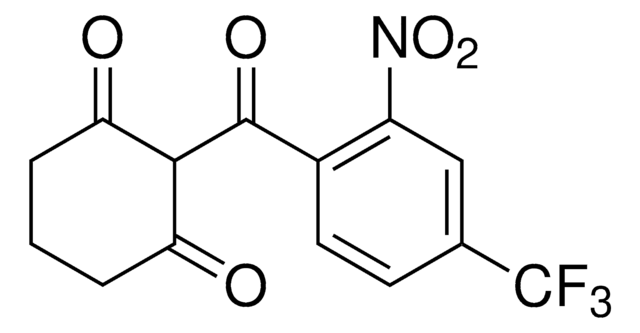
PHR1731
Nitisinone
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
Nitisinone, 2-(2-Nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione, 2-[2-Nitro-4-(trifluoromethyl)benzoyl]cyclohexane-1,3-dione, NTBC, Nitisone, SC 0735
About This Item
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
API family
nitisinone
CofA
current certificate can be downloaded
포장
pkg of 1 g
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
-10 to -25°C
SMILES string
[O-][N+](=O)c1cc(ccc1C(=O)C2C(=O)CCCC2=O)C(F)(F)F
InChI
1S/C14H10F3NO5/c15-14(16,17)7-4-5-8(9(6-7)18(22)23)13(21)12-10(19)2-1-3-11(12)20/h4-6,12H,1-3H2
InChI key
OUBCNLGXQFSTLU-UHFFFAOYSA-N
유전자 정보
human ... HPD(3242)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
생화학적/생리학적 작용
분석 메모
기타 정보
각주
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.