Y0000117
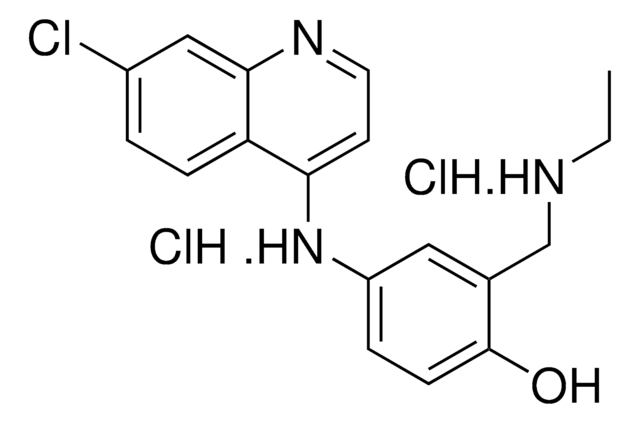
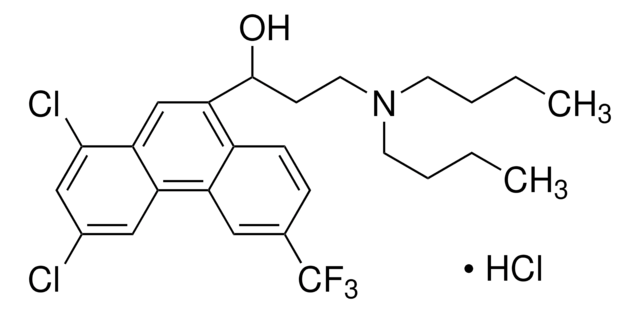
Halofantrine hydrochloride
European Pharmacopoeia (EP) Reference Standard
동의어(들):
1,3-Dichloro-a-[2-(dibutylamino)ethyl]-6-(trifluoromethyl)-9-phenanthrenemethanol hydrochloride, Halfan
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
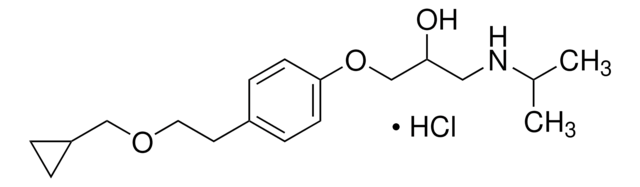
C26H30Cl2F3NO · HCl
CAS Number:
Molecular Weight:
536.88
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
halofantrine
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
Cl.CCCCN(CCCC)CCC(O)c1cc2c(Cl)cc(Cl)cc2c3cc(ccc13)C(F)(F)F
InChI
1S/C26H30Cl2F3NO.ClH/c1-3-5-10-32(11-6-4-2)12-9-25(33)23-16-22-21(14-18(27)15-24(22)28)20-13-17(26(29,30)31)7-8-19(20)23;/h7-8,13-16,25,33H,3-6,9-12H2,1-2H3;1H
InChI key
WANGFTDWOFGECH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Halofantrine hydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
Halofantrine is a blocker of delayed rectifier potassium current via the inhibition of hERG channel.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
가장 최신 버전 중 하나를 선택하세요:
Jigar P Patel et al.
Chirality, 24(7), 558-565 (2012-05-17)
Experimental hyperlipidemia has shown to decrease cytochrome P450 3A4 and 2C11 expression and to increase liver concentrations and the plasma protein binding of halofantrine (HF) enantiomers. The present study examined the effect of hyperlipidemic (HL) serum on the metabolism of
Insaf F Khalil et al.
Journal of pharmaceutical and biomedical analysis, 54(1), 168-172 (2010-09-14)
Artemether-lumefantrine (ARM-LUM) has in recent years become the first-line treatment for uncomplicated malaria in many Sub-Saharan African countries. Vigorous monitoring of the therapeutic efficacy of this treatment is needed. This requires high-quality studies following standard protocols; ideally, such studies should
René Holm et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 81(2), 281-287 (2012-04-03)
The bioavailability of the poorly soluble model drug halofantrine, dosed in a soy bean oil solution or in a self-nanoemulsifying drug delivery system (SNEDDS), at two levels of lipid, was assessed in rats. Three rat models were used: intact rats
Kerenaftali Klein et al.
The Journal of pharmacy and pharmacology, 64(11), 1603-1613 (2012-10-13)
To investigate the population pharmacokinetics of the antimalarial halofantrine (HF) in healthy volunteers and patients with symptomatic falciparum malaria. Healthy volunteer data were obtained from six volunteers who received three different doses of HF (250, 500 and 1000 mg) after
Jean-Yves Siriez et al.
Pathogens and global health, 106(2), 124-125 (2012-09-05)
In order to assess cardiac tolerance of halofantrine in children, we studied, retrospectively, 15 non complicated falciparum malaria cases treated with halofantrine, and focused on the effect on ventricular repolarisation. Our data showed that halofantrine can produce a moderate QTc
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.