Y0000724
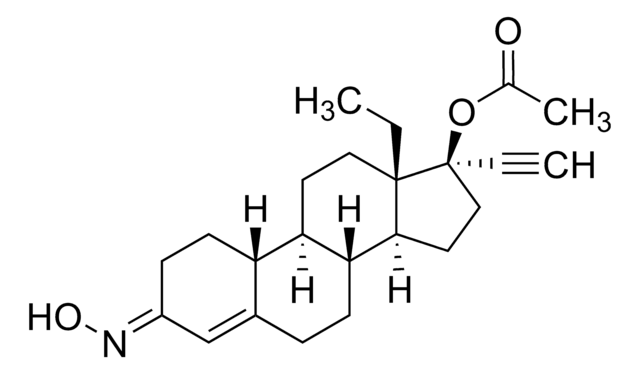
Norgestimate
European Pharmacopoeia (EP) Reference Standard
동의어(들):
(17α)-17-(Acetyloxy)-13-ethyl-18,19-dinorpregn-4-en-20-yn-3-one 3-oxime, Dexnorgestrel acetime
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C23H31NO3
CAS Number:
Molecular Weight:
369.50
Beilstein:
6440219
UNSPSC 코드:
41116107
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
norgestimate
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
CC[C@]12CC[C@H]3[C@@H](CCC4=C\C(CC[C@H]34)=N/O)[C@@H]1CC[C@@]2(OC(C)=O)C#C
InChI
1S/C23H31NO3/c1-4-22-12-10-19-18-9-7-17(24-26)14-16(18)6-8-20(19)21(22)11-13-23(22,5-2)27-15(3)25/h2,14,18-21,26H,4,6-13H2,1,3H3/b24-17+/t18-,19+,20+,21-,22-,23-/m0/s1
InChI key
KIQQMECNKUGGKA-NMYWJIRASA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Norgestimate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
M P Curran et al.
Drugs & aging, 18(11), 863-885 (2002-01-05)
The focus of this review is hormone replacement therapy (HRT) with continuous administration of micronised, oral 17beta-estradiol 1 mg/day (herein referred to as continuous estradiol) plus micronised, oral norgestimate 90 microg/day administered for 3 days then withdrawn for 3 days
J L McGuire et al.
American journal of obstetrics and gynecology, 163(6 Pt 2), 2127-2131 (1990-12-01)
Biotransformation, pharmacologic, and pharmacokinetic studies of norgestimate and its metabolites indicate that 17-deacetyl norgestimate, along with the parent drug, contributes to the biologic response. The postulated metabolic pathway, which is based on the identification of urinary products had indicated that
J Bringer
American journal of obstetrics and gynecology, 166(6 Pt 2), 1969-1977 (1992-06-01)
The efficacy and safety of a new monophasic oral contraceptive, norgestimate/ethinyl estradiol, containing the third-generation progestin, norgestimate (250 micrograms), and ethinyl estradiol (35 micrograms), are reviewed. Norgestimate/ethinyl estradiol demonstrates excellent contraceptive efficacy, with a Pearl index of 0.25. Cycle control
A Phillips et al.
American journal of obstetrics and gynecology, 167(4 Pt 2), 1191-1196 (1992-10-01)
Norgestimate is a novel progestin with highly selective progestational activity and minimal androgenicity. In rabbits, norgestimate binds to uterine progestin receptors, stimulates the endometrium, and inhibits ovulation. Norgestimate acts directly on target organs, stimulating rabbit endometrium when injected into the
S L Corson
American journal of obstetrics and gynecology, 170(5 Pt 2), 1556-1561 (1994-05-01)
Both monophasic and triphasic formulations of ethinyl estradiol plus norgestimate, a progestin with marked progesterone-receptor affinity and minimal androgen-receptor affinity, have been evaluated in numerous clinical studies designed to determine if norgestimate's receptor-binding profile provides enhanced safety without a reduction
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.