추천 제품
Grade
pharmaceutical primary standard
API family
diacerein
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
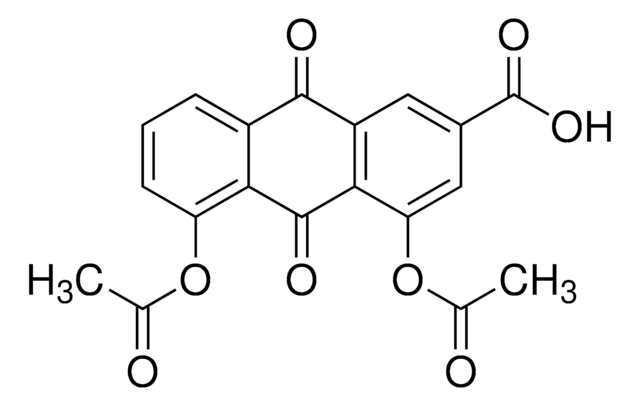
SMILES string
CC(=O)Oc1cccc2C(=O)c3cc(cc(OC(C)=O)c3C(=O)c12)C(O)=O
InChI
1S/C19H12O8/c1-8(20)26-13-5-3-4-11-15(13)18(23)16-12(17(11)22)6-10(19(24)25)7-14(16)27-9(2)21/h3-7H,1-2H3,(H,24,25)
InChI key
TYNLGDBUJLVSMA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Diacerein EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
G Falgarone et al.
Current rheumatology reports, 3(6), 479-483 (2001-11-16)
This paper reviews the most recent clinical and experimental studies on diacerein, both of which are under investigation. Diacerein could be a disease-modulating agent in osteoarthritis because structural benefits have been reported in recent trials. Moreover, after an empirical use
T S A Fidelix et al.
The Cochrane database of systematic reviews, (1)(1), CD005117-CD005117 (2006-01-27)
Osteoarthritis (OA) is one of the most prevalent musculoskeletal diseases. Diacerein acts differently from traditional non-steroidal anti-inflammatory drugs (NSAIDs) which inhibit prostaglandin synthesis, leading to adverse gastrointestinal effects. It has been proposed that diacerein acts as a slow-acting, symptom-modifying and
J P Pujol et al.
Biorheology, 37(1-2), 177-184 (2000-07-27)
The maintenance of articular cartilage integrity requires a balance between anabolic and catabolic processes which are under the control of chondrocytes. These cells are living in an anaerobic environment and normally do not divide. They are responsible for the continuous
[Diacerein].
G Passiu
Annali italiani di medicina interna : organo ufficiale della Societa italiana di medicina interna, 1(2), 172-174 (1986-06-01)
D Provvedini et al.
Presse medicale (Paris, France : 1983), 31(39 Pt 2), 4S13-4S15 (2003-01-09)
SYMPTOMATIC EFFECTS OF DIACEREIN: Data obtained from several clinical trials have demonstrated that relief of joint pain obtained with the interleukin-1 inhibitor diacerein is comparable with that observed with non-steroidal anti-inflammatory drugs (NSAIDS) after four to six weeks of treatment
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.