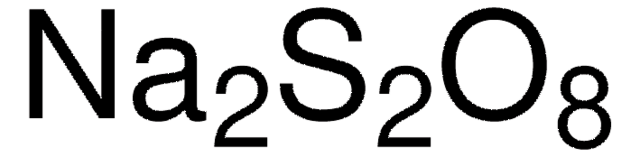모든 사진(4)
About This Item
Linear Formula:
Na2S2O8
CAS Number:
Molecular Weight:
238.10
EC Number:
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.21
분석:
≥98%
Grade:
reagent grade
양식:
powder, crystals or granules
추천 제품
Grade
reagent grade
Quality Level
분석
≥98%
양식
powder, crystals or granules
반응 적합성
reagent type: oxidant
SMILES string
[Na+].[Na+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2Na.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
InChI key
CHQMHPLRPQMAMX-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Sodium persulfate, also known as Sodium peroxodisulfate is an inorganic compound and is a water-soluble strong oxidizing agent, that is stable and easy to handle. It is commonly used as an oxidant in organic synthesis reactions such as transition-metal-catalyzed or metal-free reactions. Additionally, it is used as a polymerization initiator and in the synthesis of sulfate radical anions through heat-activated decomposition.
애플리케이션
Sodium persulfate can be used as an oxidant:
- For the radical cyclization cascade reaction of 2-alkynylbenzonitriles and sodium arylsulfinates to synthesize sulfonated indenones.
- In the silica-supported aluminum chloride-catalyzed Baeyer-Villiger oxidation of cyclic and acyclic ketones to lactones or esters.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
PCE oxidation by sodium persulfate in the presence of solids
Jed C, et al.
Environmental Science & Technology, 44, 9445-9450 (2010)
Esther Eljarrat-Binstock et al.
Investigative ophthalmology & visual science, 45(8), 2543-2548 (2004-07-28)
To assess the corneal iontophoretic delivery of gentamicin by drug-loaded hydrogel probe, and to determine the resultant ocular disposition and elimination of the drug from the cornea and anterior chamber. Corneal iontophoresis of gentamicin sulfate was studied in healthy white
Xiaodan Zhao et al.
Journal of the American Chemical Society, 132(16), 5837-5844 (2010-04-03)
By palladium catalysis, the C-H bond functionalization of O-phenylcarbamates with simple arenes has been achieved using sodium persulfate (Na(2)S(2)O(8)), an inexpensive, easy-to-handle, and environmentally friendly oxidant. This oxidative cross-coupling involves two aromatic C-H bonds undergoing concomitant oxidation to furnish a
Chenju Liang et al.
Chemosphere, 70(3), 426-435 (2007-08-19)
In situ chemical oxidation with persulfate anion (S2O82*) is a viable technique for remediation of groundwater contaminants such as trichloroethylene (TCE). An accelerated reaction using S2O82* to destroy TCE can be achieved via chemical activation with ferrous ion to generate
N Sabri et al.
The Science of the total environment, 427-428, 382-389 (2012-05-15)
The objective of this work was to evaluate the removal of ibuprofen (IBP) using the oxidants hydrogen peroxide (H(2)O(2)) and sodium persulfate (Na(2)S(2)O(8)). The ability of magnetite (Fe(3)O(4)) to activate persulfate (PS) and H(2)O(2) for the oxidation of IBP at
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.