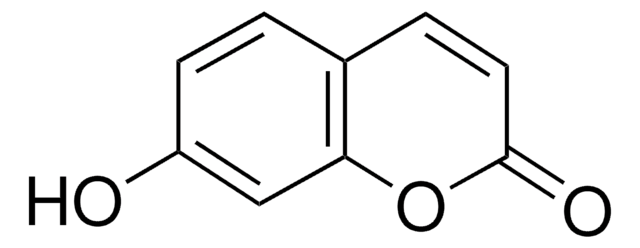
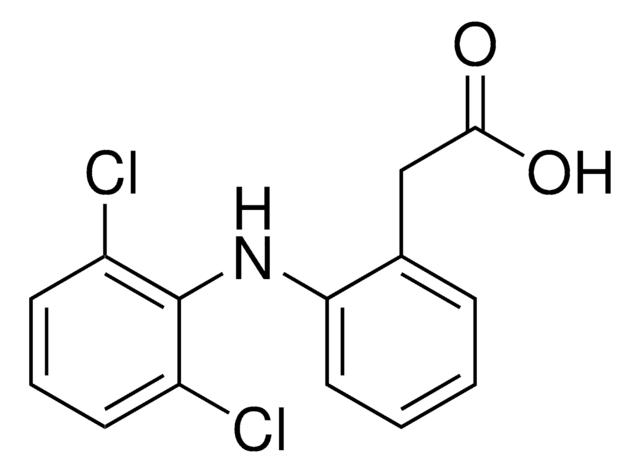
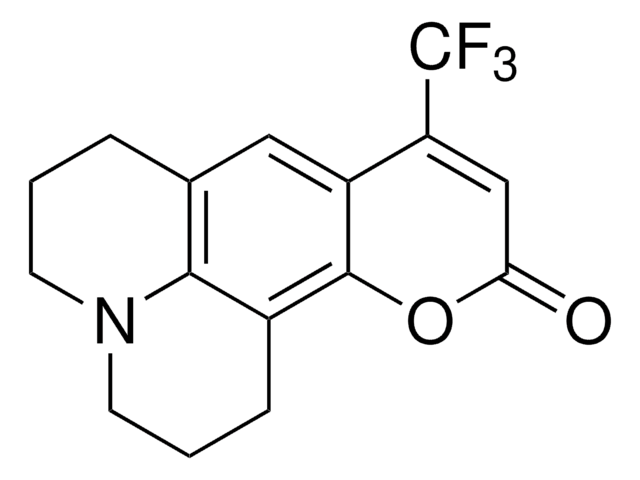
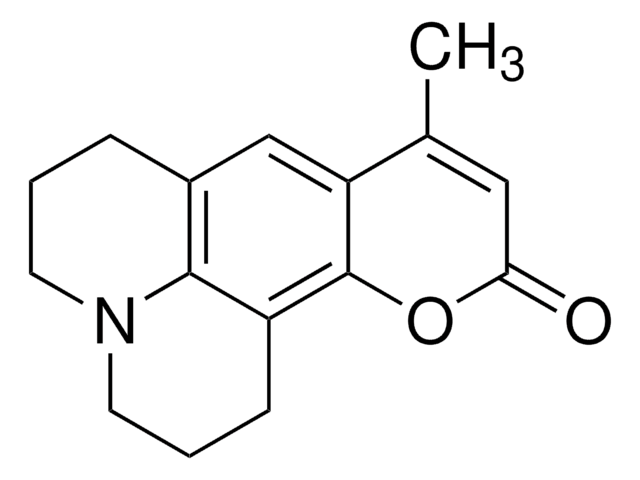
추천 제품
vapor pressure
0.01 mmHg ( 47 °C)
Quality Level
분석
≥99% (HPLC)
양식
powder
bp
298 °C (lit.)
mp
68-73 °C (lit.)
SMILES string
O=C1Oc2ccccc2C=C1
InChI
1S/C9H6O2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-6H
InChI key
ZYGHJZDHTFUPRJ-UHFFFAOYSA-N
유전자 정보
rat ... Maoa(29253) , Maob(25750)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Coumarin is useful as a precursor molecule for the preparation or synthesis of coumarin-based anticoagulates, antiiflammatory agents and antioxidation superoxide scavengers.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point (°F)
323.6 °F - closed cup
Flash Point (°C)
162 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Klaus Abraham et al.
Molecular nutrition & food research, 54(2), 228-239 (2009-12-22)
Coumarin is a secondary phytochemical with hepatotoxic and carcinogenic properties. For the carcinogenic effect, a genotoxic mechanism was considered possible, but was discounted by the European Food Safety Authority in 2004 based on new evidence. This allowed the derivation of
Donglei Yu et al.
Medicinal research reviews, 23(3), 322-345 (2003-03-21)
Numerous plant-derived compounds have been evaluated for inhibitory effects against HIV replication, and some coumarins have been found to inhibit different stages in the HIV replication cycle. This review article describes recent progress in the discovery, structure modification, and structure-activity
María E Riveiro et al.
Bioorganic & medicinal chemistry, 17(18), 6547-6559 (2009-09-01)
The presumption that some coumarins might be lead compounds in the search for new differentiation agents against leukemia is based on the fact that natural coumarins, 5-(3-methyl-2-butenyloxy)-6,7-methylenedioxycoumarin (C-2) and 5-methoxy-6,7-methylenedioxycoumarin (C-1) inhibit proliferation and induce differentiation in U-937 cells [Riveiro
Shigehiro Sumiya et al.
The journal of physical chemistry. A, 117(7), 1474-1482 (2013-01-25)
A coumarin-amide-dipicolylamine linkage (L) was synthesized and used as a fluorescent receptor for metal cations in water. The receptor dissolved in water with neutral pH shows almost no fluorescence due to the photoinduced electron transfer (PET) from the amide and
Shota Morimoto et al.
Chemical communications (Cambridge, England), 49(18), 1811-1813 (2013-01-26)
A photo-switchable fluorescent flagging approach has been developed to identify photoaffinity-labeled peptides in target protein. Upon photochemical release of the ligand, the protein was newly modified with a coumarin in place of the previously attached biotin. It allowed us to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.