추천 제품
생물학적 소스
bovine spleen
Quality Level
유형
Type X
분석
>25% protein (biuret)
형태
lyophilized powder
특이 활성도
≥5 units/mg protein
구성
Protein, ≥25% biuret
제조업체/상표
Sigma-Aldrich
저장 조건
OK to freeze (Unstable. Keep frozen)
농도
≥5 unit/mg protein
기술
activity assay: suitable
적합성
suitable for molecular biology
응용 분야
life science and biopharma
배송 상태
dry ice
저장 온도
−20°C
유전자 정보
cow ... CTSC(352958)
일반 설명
Research Area: Cell Signaling
Dipeptidyl peptidase I (DPPI), also known as cathepsin C, is an abundant lysosomal cysteine protease from the papain superfamily with a molecular weight of approximately 200 kDa. It is widely expressed in a variety of mammalian tissues, with the highest levels found in the lungs, kidneys, liver, and spleen, and relatively lower levels in the brain.
DPPI is the only member of its family that is functional as a tetramer, consisting of four identical subunits, each composed of an N-terminal fragment, a heavy chain, and a light chain. It is identified as one of the multifaceted protease-processing machines, having been shown to function beyond its role as a non-specific lysosomal protease.
Dipeptidyl peptidase I (DPPI), also known as cathepsin C, is an abundant lysosomal cysteine protease from the papain superfamily with a molecular weight of approximately 200 kDa. It is widely expressed in a variety of mammalian tissues, with the highest levels found in the lungs, kidneys, liver, and spleen, and relatively lower levels in the brain.
DPPI is the only member of its family that is functional as a tetramer, consisting of four identical subunits, each composed of an N-terminal fragment, a heavy chain, and a light chain. It is identified as one of the multifaceted protease-processing machines, having been shown to function beyond its role as a non-specific lysosomal protease.
애플리케이션
Cathepsin C from bovine spleen has been used for the in vitro enzyme activity assays. It has also been used as a digestion enzyme for in vitro myelin oligodendrocyte glycoprotein (MOG) digestion.
Cathepsin C has been used in a study that demonstrated the potential of a proteomics approach to identify novel proteins expressed by extravillous trophoblast and to uncover the mechanisms leading to disease states in pregnancy. Cathepsin C has also been used in a study to evaluate biodegradable thermogels.
The enzyme from Sigma has been used in the activation of granzyme k (Gzmk) precursor from E. coli. Granzymes are granule-stored lymphocyte serine proteases that are implicated in T- and natural killer cell-mediated cytotoxic defense reactions.
생화학적/생리학적 작용
Cathepsin C (Cat C) serves as the physiological activator of groups of serine proteases within immune and inflammatory cells, playing a crucial role in the defense mechanisms of an organism. It may play a role in chronic airway diseases such as asthma. Cat C also acts as a protease link between inflammation and thrombosis.
Cat C participates in neutrophil recruitment and production of chemokines and cytokines in many inflammatory diseases. Cathepsin C plays a crucial role as an essential enzyme in activating granule serine proteases in cytotoxic T lymphocytes, natural killer cells (granzymes A and B), mast cells (chymase and tryptase), and neutrophils (cathepsin G, proteinase 3, and elastase).
Cat C participates in neutrophil recruitment and production of chemokines and cytokines in many inflammatory diseases. Cathepsin C plays a crucial role as an essential enzyme in activating granule serine proteases in cytotoxic T lymphocytes, natural killer cells (granzymes A and B), mast cells (chymase and tryptase), and neutrophils (cathepsin G, proteinase 3, and elastase).
Cathespin C is a dipeptidyl aminopeptidase that can sequentially remove dipeptides from a peptide chain with an unsubstituted N-terminus. The enzyme exhibits a preference for glycine and proline as N-terminal aminoacids. Substrates that have an N-terminal lysyl or arginyl residue, or a penultimate proryl residue are not targeted by this enzyme. The endopeptidase activity requires the presence of halide ions and sulfydryl activators.
주의사항
Unstable. Keep frozen.
단위 정의
One unit will produce 1 μmole of Gly-Phe-NHOH from Gly-Phe-NH2 and hydroxylamine per min at pH 6.8 at 37 °C using DL-phenylalanine hydroxamic acid as the standard. In addition to its hydrolytic properties, cathepsin C catalyzes the polymerization of dipeptide amides.
물리적 형태
Lyophilized from a 1 M sodium chloride solution.
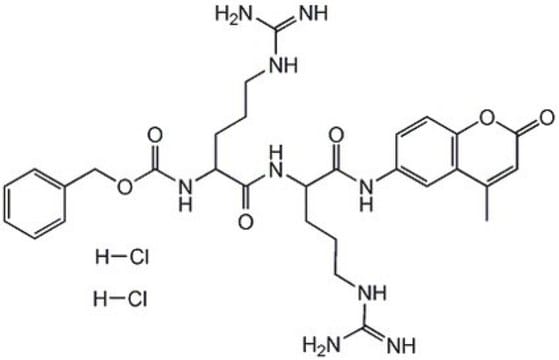
기질
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Case of rippled-pattern sebaceoma with clinically yellowish surface and histopathological paucity of lipid-containing neoplastic cells.
Yoshio Kawakami et al.
The Journal of dermatology, 39(7), 644-646 (2011-11-15)
Up-regulation of microglial cathepsin C expression and activity in lipopolysaccharide-induced neuroinflammation
Fan K, et al.
Journal of Neuroinflammation, 9, 1-13 (2012)
Mayumi Ueta et al.
Japanese journal of ophthalmology, 55(4), 405-410 (2011-05-28)
We previously reported that human conjunctival epithelial cells expressed functioning interleukin-4 receptor α (IL-4Rα). In this study, we investigated whether human corneal epithelial cells also express functioning IL-4Rα. The presence of IL-4Rα mRNA and protein in human corneal epithelium was
ClC-7 drives intraphagosomal chloride accumulation to support hydrolase activity and phagosome resolution
Wu JZ, et al.
The Journal of cell biology, 222(6), e202208155-e202208155 (2023)
Christian D Sadik et al.
Clinical oral investigations, 16(2), 591-597 (2011-03-08)
Papillon-Lefèvre syndrome (PLS) is characterised by aggressively progressive periodontitis combined with palmo-plantar hyperkeratosis. It is caused by "loss of function" mutations in the cathepsin C gene. The hypothesis behind this study is that PLS patients' polymorphonuclear leukocytes (PMNs) produce more
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.