H4381
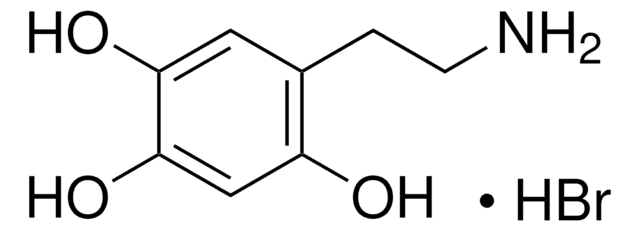
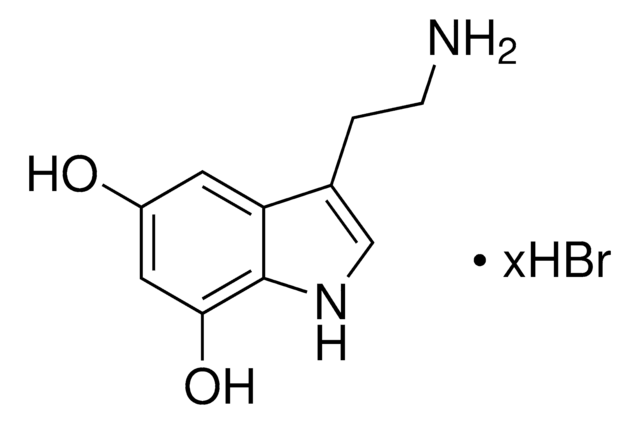
6-Hydroxydopamine hydrochloride
≥97% (titration), powder, neurotoxin
동의어(들):
2,4,5-Trihydroxyphenethylamine hydrochloride, 2,5-Dihydroxytyramine hydrochloride, 2-(2,4,5-Trihydroxyphenyl)ethylamine hydrochloride, 6-OHDA
로그인조직 및 계약 가격 보기
모든 사진(5)
About This Item
Linear Formula:
(HO)3C6H2CH2CH2NH2 · HCl
CAS Number:
Molecular Weight:
205.64
Beilstein:
4274007
EC Number:
MDL number:
UNSPSC 코드:
12352116
PubChem Substance ID:
NACRES:
NA.77
추천 제품
제품명
6-Hydroxydopamine hydrochloride, ≥97% (titration), powder
Quality Level
분석
≥97% (titration)
양식
powder
색상
off-white to brown
mp
232-233 °C (dec.) (lit.)
solubility
H2O: >50 mg/mL, clear, yellow to brown
저장 온도
room temp
SMILES string
Cl.NCCc1cc(O)c(O)cc1O
InChI
1S/C8H11NO3.ClH/c9-2-1-5-3-7(11)8(12)4-6(5)10;/h3-4,10-12H,1-2,9H2;1H
InChI key
QLMRJHFAGVFUAC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
6-Hydroxydopamine hydrochloride (6-OHDA) is a neurotoxin, commonly used to induce Parkinson′s disease (PD). 6-OHDA causes death of dopaminergic neurons in substantia nigra pars compacta progressively mimicking PD. 6-OHDA is highly oxidizable and cannot cross blood brain barrier. 6-OHDA exerts cytotoxicity by generating reactive oxygen species, initiating cellular stress and cell death. 6-OHDA leads to neuronal cell death in many in vitro models like primary neuronal culture, human neuroblastoma cell line and rat adrenal pheochromocytoma cell line, PC12.
애플리케이션
6-hydroxydopamine hydrochloride has been used:
- to induce Parkinson′s disease (PD) in rats
- to analyse cytotoxic effect of 6-OHDA on PC12 cell line
- to induce noradrenergic (NA) neuron deletion from the locus-coeruleus
생화학적/생리학적 작용
Neurotoxin that destroys catecholaminergic terminals.
주의사항
Hygroscopic
재구성
Dissolve in oxygen-free water containing 0.1% sodium metabisulfite or other antioxidants.
Solutions should be freshly prepared and protected from exposure to light. Solutions turn red as it oxidizes.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Human induced pluripotent stem cell-derived neurons improve motor asymmetry in a 6-hydroxydopamine-induced rat model of Parkinson's disease
Han F, et al.
Cytotherapy, 17(5), 665-679 (2015)
Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: involvement of hydrogen peroxide-dependent and-independent action
Saito Y, et al.
Free Radical Biology & Medicine, 42(5), 675-685 (2007)
The 6-hydroxydopamine model and parkinsonian pathophysiology: Novel findings in an older model
Hernandez-Baltazar D, et al.
Neurologia (Barcelona, Spain), 32(8), 533-539 (2017)
Kryspin Andrzejewski et al.
Journal of biomedical science, 24(1), 24-24 (2017-03-30)
Malfunctioning of the serotonergic system in Parkinson's disease may contribute to non-motor symptoms such as respiratory complications. Thus the aim of our study was to investigate the role of serotonin 5-HT Wistar rats were lesioned unilaterally with double 6-hydroxydopamine (6-OHDA)
Víctor Fernández-Dueñas et al.
International journal of molecular sciences, 20(14) (2019-07-26)
Background: Several biophysical techniques have been successfully implemented to detect G protein-coupled receptors (GPCRs) heteromerization. Although these approaches have made it possible to ascertain the presence of GPCR heteromers in animal models of disease, no success has been accomplished in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.