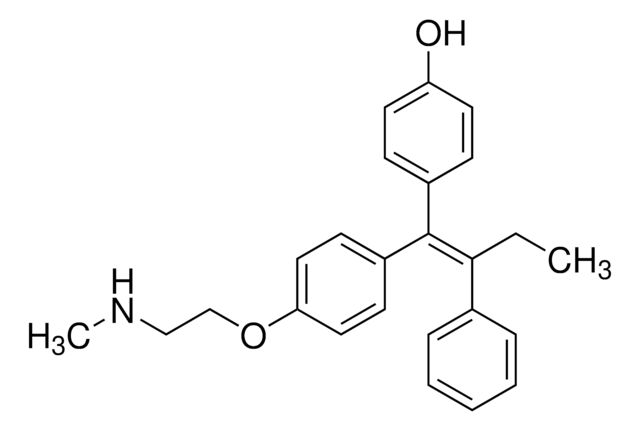
H6278
4-Hydroxytamoxifen
≥98% (HPLC), powder, Tamoxifen metabolite
동의어(들):
4-(1-[4-(Dimethylaminoethoxy)phenyl]-2-phenyl-1-butenyl)phenol, 4-OHT, cis/trans-4-Hydroxytamoxifen
About This Item
추천 제품
제품명
4-Hydroxytamoxifen, ≥70% Z isomer (remainder primarily E-isomer)
Quality Level
분석
≥98% (HPLC)
양식
powder
저장 조건
desiccated
protect from light
solubility
methanol: 10 mg/mL
ethanol: 20 mg/mL (with heating)
항생제 활성 스펙트럼
neoplastics
동작 모드
enzyme | inhibits
주관자
AstraZeneca
저장 온도
2-8°C
SMILES string
CC\C(c1ccccc1)=C(/c2ccc(O)cc2)c3ccc(OCCN(C)C)cc3.CC\C(c4ccccc4)=C(\c5ccc(O)cc5)c6ccc(OCCN(C)C)cc6
InChI
1S/2C26H29NO2/c2*1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h2*5-17,28H,4,18-19H2,1-3H3/b26-25+;26-25-
InChI key
ZJLDABGSDWXVGE-BDSXMVAQSA-N
유전자 정보
human ... ESR1(2099) , ESR2(2100) , ESRRG(2104) , IL6(3569)
rat ... Ar(24208) , Esr1(24890) , Esr2(25149)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4-OHT effectively inhibited cell growth in the absence of estrogen when cell proliferation was stimulated by insulin or epidermal growth factor. 4-OHT inhibits lipid peroxidation within cell membranes and shows peroxyl radical scavenging activity.
애플리케이션
- to induce the recombination of small intestinal organoids.
- to study its impact on the ability of human peripheral blood mononuclear cells (PMNCs) to form hematopoietic colonies.
- to induce overexpression of MYCN in the neuroblastoma cell line to determine how the elevated MYCN expression influences the sensitivity of neuroblastoma cells to BIRC5/survivin inhibitor, YM155-induced apoptosis.
생화학적/생리학적 작용
특징 및 장점
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
이미 열람한 고객
문서
We presents an article on Autophagy in Cancer Promotes Therapeutic Resistance
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.