모든 사진(3)
About This Item
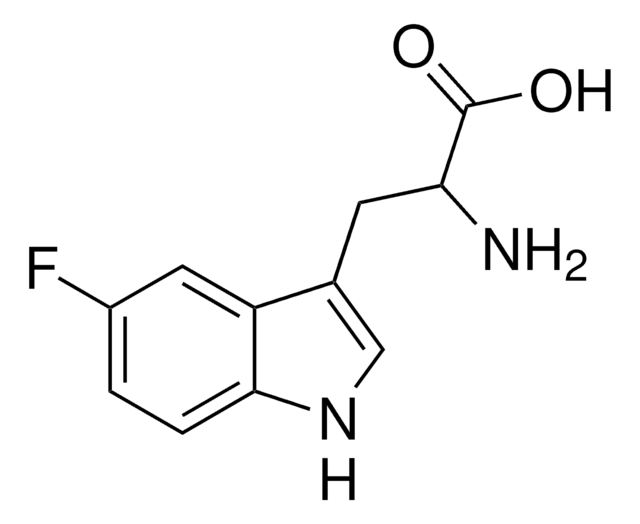
실험식(Hill 표기법):
C12H14N2O3
CAS Number:
Molecular Weight:
234.25
Beilstein:
26781
EC Number:
MDL number:
UNSPSC 코드:
12352209
PubChem Substance ID:
NACRES:
NA.26
추천 제품
제품명
5-Methoxy-DL-tryptophan,
분석
≥98% (TLC)
Quality Level
양식
crystalline
색상
white to light beige
mp
258-261 °C (dec.) (lit.)
응용 분야
peptide synthesis
저장 온도
−20°C
SMILES string
COc1ccc2[nH]cc(CC(N)C(O)=O)c2c1
InChI
1S/C12H14N2O3/c1-17-8-2-3-11-9(5-8)7(6-14-11)4-10(13)12(15)16/h2-3,5-6,10,14H,4,13H2,1H3,(H,15,16)
InChI key
KVNPSKDDJARYKK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
생화학적/생리학적 작용
5-Methoxy-DL-tryptophan is an amino acid derivative.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
J van Benthem et al.
Journal of neural transmission, 61(3-4), 219-237 (1985-01-01)
Until now the day/night and seasonal rhythmicity in the synthesis of 5-methoxyindoles (MI) is thought to be regulated by environmental factors, especially photoperiod and temperature. Endogenous factors are also implicated in the generation of N-acetyltransferase and hydroxyindole-O-methyltransferase activity rhythms. In
J van Benthem et al.
Journal of neural transmission, 67(1-2), 147-162 (1986-01-01)
Testes weight, plasma FSH and LH concentration and pineal methylating capacity were compared in hamsters housed under either long (LD14:10) or short (LD8:16) photoperiods. Hamsters housed for 14 weeks under short photoperiod showed gonadal atrophy, which was complete after 6
Anika Kremer et al.
Applied microbiology and biotechnology, 79(6), 951-961 (2008-05-16)
Recently, a gene for a 7-dimethylallyltryptophan synthase (7-DMATS) was identified in Aspergillus fumigatus and its enzymatic function was proven biochemically. In this study, the behaviour of 7-DMATS towards aromatic substrates was investigated and compared with that of the 4-dimethylallyltryptophan synthase
M Leino et al.
Medical biology, 63(4), 160-169 (1985-01-01)
Melatonin and other 5-methoxyindoles are compounds usually associated with the pineal gland. Research is expanding from studies of pineal melatonin to studies of extrapineal organs and of other 5-methoxyindoles besides melatonin. Research in recent years has shown that the retina
M G Balemans et al.
Journal of neural transmission, 58(1-2), 121-134 (1983-01-01)
The pineals of 28 days old male Wistar rats, in December periodically exposed to either white or green light, were incubated with pterin-6-aldehyde or reduced neopterin. In white light the rhythm of synthesis of 5-methoxytryptophan and of 5-methoxyindole-3-acetic acid was
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.