모든 사진(1)
About This Item
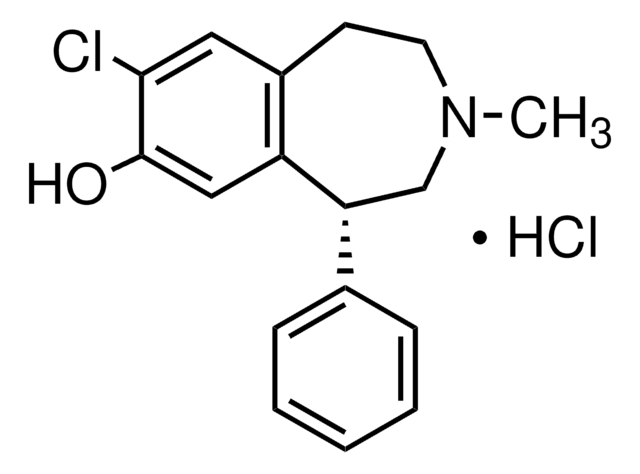
실험식(Hill 표기법):
C16H17NO2 · HCl
CAS Number:
Molecular Weight:
291.77
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
solid
광학 활성
[α]22/D +16.1°, c = 1.2 in methanol(lit.)
저장 조건
desiccated
색상
off-white to light tan
solubility
0.1 M HCl: 1.2 mg/mL
ethanol: 3.4 mg/mL
H2O: 5 mg/mL
aqueous base: unstable
SMILES string
Cl.Oc1cc2CCNC[C@H](c3ccccc3)c2cc1O
InChI
1S/C16H17NO2.ClH/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11;/h1-5,8-9,14,17-19H,6-7,10H2;1H/t14-;/m1./s1
InChI key
YEWHJCLOUYPAOH-PFEQFJNWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
(R)-(+)-SKF-38393 hydrochloride has been used as a D1 dopamine receptor agonist to study its effect on the sleep-wake pattern of macaques rendered parkinsonian with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
생화학적/생리학적 작용
(R)-(+)-SKF-38393 hydrochloride is a D1 dopamine receptor agonist and an active enantiomer of (±)-SKF-38393. It enhances pertussis toxin-insensitive and protein kinase A-mediated glutamate release in the hippocampal neurons. SKF-38393, a benzazepine derivative, exhibits anorectic effects.
특징 및 장점
This compound is featured on the Dopamine Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
주의사항
Light sensitive
재구성
Solutions may be stored for several days at 4 °C.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
M R Zarrindast et al.
Pharmacology, 87(1-2), 85-89 (2011-01-19)
Both the dopamine receptor D(1) agonist SKF 38393 and the antagonist SCH 23390 are benzazepine derivatives that have been widely used as pharmacological tools and radioligands. Evidence suggests that behavioral effects of both compounds do not always correspond to their
Carole Hyacinthe et al.
Neurobiology of disease, 63, 20-24 (2013-11-12)
Both excessive daytime sleepiness (EDS) and rapid eye movement (REM) sleep deregulation are part of Parkinson's disease (PD) non-motor symptoms and may complicate dopamine replacement therapy. We report here that dopamine agonists act differentially on sleep architecture in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
A Bouron et al.
Neuroscience, 94(4), 1063-1070 (2000-01-07)
The present study was undertaken to better assess the role of dopamine on exocytosis. Since direct activation of adenylate cyclase (e.g., with forskolin) enhances neurotransmitter release it was of interest to see whether the activation of D1-type dopamine receptors, which
C Kaiser et al.
Journal of medicinal chemistry, 25(6), 697-703 (1982-06-01)
Resolution of the unique dopamine receptor agonist 2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepine (1) was achieved by a stereospecific multistep conversion of the readily separated enantiomers of its O,O,N-trimethylated precursor 2. The absolute stereochemistry of the antipodes of 2-MeI was determined by single-crystal X-ray diffractometric
Steven J Cooper et al.
European journal of pharmacology, 532(3), 253-257 (2006-02-16)
Free-feeding rats meet much of their daily energy requirements by consuming food in meals during the nocturnal phase of the night/day cycle. Meal pattern analysis methodology has been developed to record the patterns of meal taken over a 24-h period
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.