추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
저장 조건
desiccated
색상
yellow
solubility
DMSO: ≥15 mg/mL
주관자
Roche
저장 온도
2-8°C
SMILES string
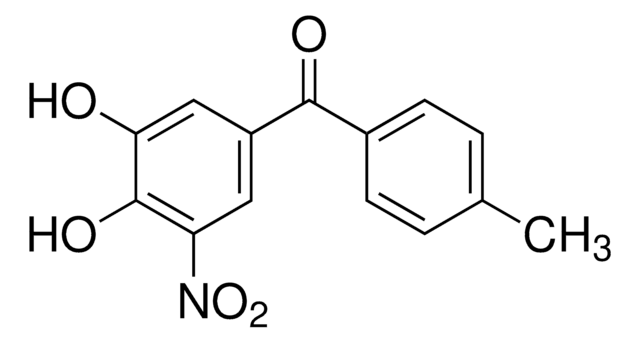
Cc1ccc(cc1)C(=O)c2cc(O)c(O)c(c2)[N+]([O-])=O
InChI
1S/C14H11NO5/c1-8-2-4-9(5-3-8)13(17)10-6-11(15(19)20)14(18)12(16)7-10/h2-7,16,18H,1H3
InChI key
MIQPIUSUKVNLNT-UHFFFAOYSA-N
유전자 정보
human ... COMT(1312)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Tolcapone may be used in COMT-mediated cell signaling studies.
Tolcapone has been used in methyltransferase assay in human embryonic kidney 293 cells.
Tolcapone has been used in methyltransferase assay in human embryonic kidney 293 cells.
생화학적/생리학적 작용
Inhibition of catechol-O-methyltransferase by tolcapone increases the half-life and bioavailabilty of levodopa. It therefore is an effective adjunctive therapy in patients with Parkinson′s disease with motor response fluctuations.
Orally active catechol-O-methyltransferase (COMT) inhibitor: inhibits both central and peripheral COMT.
Tolcapone is an orally active catechol-O-methyltransferase (COMT) inhibitor. It inhibits both central and peripheral COMT. Tolcapone crosses the blood-brain barrier, and has been used for L-DOPA adjunct therapy in the treatment of Parkinson′s Disease.
특징 및 장점
This compound was developed by Roche. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Acute 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Jenny J Fischer et al.
Toxicological sciences : an official journal of the Society of Toxicology, 113(1), 243-253 (2009-09-29)
Capture compound mass spectrometry (CCMS) is a novel technology that helps understand the molecular mechanism of the mode of action of small molecules. The Capture Compounds are trifunctional probes: A selectivity function (the drug) interacts with the proteins in a
Daniel D Truong
Clinical interventions in aging, 4, 109-113 (2009-06-09)
Levodopa has been the gold standard therapy for the motor symptoms of Parkinson's disease for more than three decades. Although it remains the most effective treatment, its long-term use is associated with motor fluctuations and dyskinesias that can be disabling
Martha Kimos et al.
Journal of biomolecular screening, 21(5), 490-495 (2015-11-20)
Catechol-O-methyltransferase (COMT) plays an important role in the deactivation of catecholamine neurotransmitters and hormones. Inhibitors of COMT, such as tolcapone and entacapone, are used clinically in the treatment of Parkinson's disease. Discovery of novel inhibitors has been hampered by a
Saviana Di Giovanni et al.
The Journal of biological chemistry, 285(20), 14941-14954 (2010-02-13)
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease (AD). There is considerable consensus that the increased production and/or aggregation of alpha-synuclein (alpha-syn) plays a central role in the pathogenesis of PD and related synucleinopathies. Current
Inès Khsime et al.
International journal of molecular sciences, 24(19) (2023-10-14)
L-DOPA, the precursor of catecholamines, exerts a pro-locomotor action in several vertebrate species, including newborn rats. Here, we tested the hypothesis that decreasing the degradation of monoamines can promote the pro-locomotor action of a low, subthreshold dose of L-DOPA in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.