추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
white to beige
solubility
DMSO: 5 mg/mL (clear solution)
배송 상태
wet ice
저장 온도
−20°C
SMILES string
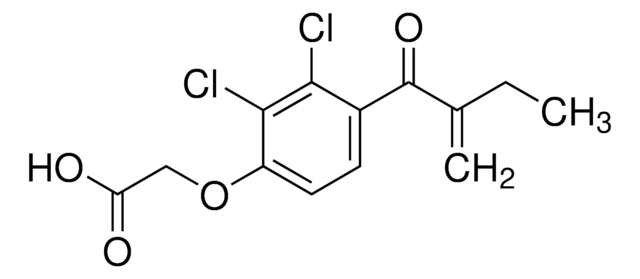
OC(=O)COc1ccc(c(Cl)c1Cl)C(=O)c2cccs2
InChI
1S/C13H8Cl2O4S/c14-11-7(13(18)9-2-1-5-20-9)3-4-8(12(11)15)19-6-10(16)17/h1-5H,6H2,(H,16,17)
InChI key
AGHANLSBXUWXTB-UHFFFAOYSA-N
생화학적/생리학적 작용
Tienilic Acid (Ticrynafen) is a P450 inhibitor, a specific suicide substrate for CYP2C9 and CYP2C10. It was once used as a loop diuretic drug with uric acid-lowering (uricosuric) actvity, but was removed from market because of hepatotoxicity.
Tienilic Acid is a P450 inhibitor, a specific suicide substrate for CYP2C9 and CYP2C10.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Use of isotopes and LC-MS-ESI-TOF for mechanistic studies of tienilic acid metabolic activation.
M Belghazi et al.
Advances in experimental medicine and biology, 500, 139-144 (2002-01-05)
Daisuke Satoh et al.
PloS one, 12(10), e0187072-e0187072 (2017-10-25)
The utility of HepG2 cells to assess drug metabolism and toxicity induced by chemical compounds is hampered by their low cytochrome P450 (CYP) activities. To overcome this limitation, we established HepG2 cell lines expressing major CYP enzymes involved in drug
Peter M Rademacher et al.
Chemical research in toxicology, 25(4), 895-903 (2012-02-15)
The uricosuric diuretic agent tienilic acid (TA) is a thiophene-containing compound that is metabolized by P450 2C9 to 5-OH-TA. A reactive metabolite of TA also forms a covalent adduct to P450 2C9 that inactivates the enzyme and initiates immune-mediated hepatic
Hideo Takakusa et al.
Drug metabolism and disposition: the biological fate of chemicals, 36(5), 816-823 (2008-01-30)
The metabolic activation of a drug to an electrophilic reactive metabolite and its covalent binding to cellular macromolecules is considered to be involved in the occurrence of idiosyncratic drug toxicity (IDT). As a cellular defense system against oxidative and electrophilic
M Pilar López-García et al.
Biochemical pharmacology, 70(12), 1870-1882 (2005-11-01)
Drug-induced autoimmune hepatitis is among the most severe hepatic idiosyncratic adverse drug reactions. Considered multifactorial, the disease combines immunological and metabolic aspects, the latter being to date much better known. As for many other model drugs, studies on tienilic acid
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.