모든 사진(1)
About This Item
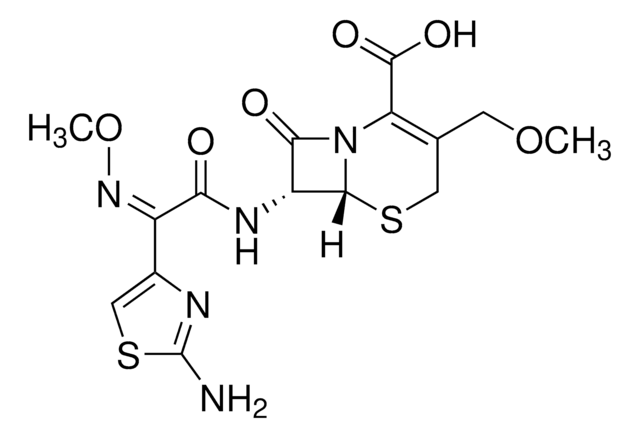
실험식(Hill 표기법):
C21H27N5O9S2
CAS Number:
Molecular Weight:
557.60
MDL number:
UNSPSC 코드:
41116107
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
cefpodoxime
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
[s]1c(nc(c1)\C(=N\OC)\C(=O)N[C@H]2[C@H]3SCC(=C(N3C2=O)C(=O)OC(OC(=O)OC(C)C)C)COC)N
InChI
1S/C21H27N5O9S2/c1-9(2)33-21(30)35-10(3)34-19(29)15-11(6-31-4)7-36-18-14(17(28)26(15)18)24-16(27)13(25-32-5)12-8-37-20(22)23-12/h8-10,14,18H,6-7H2,1-5H3,(H2,22,23)(H,24,27)/b25-13-/t10?,14-,18-/m1/s1
InChI key
LTINZAODLRIQIX-FBXRGJNPSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Cefpodoxime proxetil USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Cefpodoxime Proxetil for Oral Suspension
- Cefpodoxime Proxetil Tablets
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Amrita Bajaj et al.
Drug development and industrial pharmacy, 39(5), 635-645 (2012-05-09)
Lipid based drug delivery systems have gained prominence in last decade for drugs with dissolution rate limited oral bioavailability. To improve the solubility, permeability and oral bioavailability of cefpodoxime proxetil, β-lactam antibiotic. It is BCS Class IV drug having solubility
Abhijit A Date et al.
International journal of pharmaceutics, 329(1-2), 166-172 (2006-10-03)
Self-nanoemulsifying drug delivery systems (SNEDDS) were developed with the objective to overcome problems associated with the delivery of cefpodoxime proxetil (CFP), a poorly bioavailable high dose antibiotic having pH dependant solubility. Solubility of CFP in oily phases and surfactants was
Vasu Kumar Kakumanu et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 64(2), 255-259 (2006-06-27)
Cefpodoxime proxetil (CP) is a prodrug of cefpodoxime acid (CA), and is supplied as racemic mixture of R- and S-enantiomers. CP has only 50% absolute bioavailability, and the reasons responsible for low bioavailability remain poorly understood. The present work ascertains
S Bhargava et al.
Current drug delivery, 5(1), 1-6 (2008-01-29)
Cefpodoxime proxetil (CPDX-PR) is an oral cephalosporin antibiotic with poor aqueous solubility and bioavailability. Effect of beta-cyclodextrin on aqueous solubility and dissolution rate of cefpodoxime proxetil was evaluated by the formation of solid inclusion complexes in 1:2 molar ratio of
Md Salim Shakur et al.
Indian pediatrics, 44(11), 838-841 (2007-12-07)
In order to evaluate clinical and bacteriological efficacy of Cefpodoxime Proxetil (CP) in typhoid fever in comparison to cefixime (CF), we assessed 140 children with suspected typhoid fever. Fulfilling inclusion criteria finally 40 culture confirmed typhoid fever were allocated in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.