1224981
USP
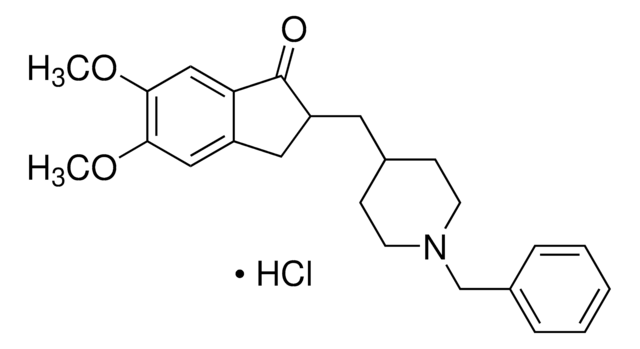
Donepezil hydrochloride
United States Pharmacopeia (USP) Reference Standard
동의어(들):
(±)-2-[(1-Benzyl-4-piperidyl)methyl]-5,6-dimethoxy-1-indanone hydrochloride, 2,3-Dihydro-5,6-dimethoxy-2-{[1-(phenylmethyl)-4-piperidinyl]methyl}-1H-inden-1-one
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C24H29NO3 · HCl
CAS Number:
Molecular Weight:
415.95
MDL number:
UNSPSC 코드:
41116107
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
donepezil
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
Cl.N2(CCC(CC2)CC3Cc4c(cc(c(c4)OC)OC)C3=O)Cc1ccccc1
InChI
1S/C24H29NO3.ClH/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18;/h3-7,14-15,17,20H,8-13,16H2,1-2H3;1H
InChI key
XWAIAVWHZJNZQQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Oral - Eye Irrit. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Hachiro Sugimoto et al.
Japanese journal of pharmacology, 89(1), 7-20 (2002-06-27)
A wide range of evidence shows that cholinesterase (ChE) inhibitors can interfere with the progression of Alzheimer's disease (AD). The earliest known ChE inhibitors, namely, physostigmine and tacrine, showed modest improvement in the cognitive function of AD patients. However, clinical
Kenneth Rockwood et al.
The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques, 40(4), 564-571 (2013-06-22)
vascular dementia (VaD) and mixed Alzheimer's disease (AD/VaD) are common. How best to monitor treatment is not clear. Our objective was to compare responsiveness and construct validity of change scores, following donepezil treatment, of the standardized Mini-Mental State Examination (sMMSE)
Jong-Il Kim et al.
International journal of pharmaceutics, 455(1-2), 31-39 (2013-08-13)
Although the taste-masking of bitter drug using ion exchange resin has been recognized, in vitro testing using an electronic tongue (e-Tongue) and in vivo bitterness test by human panel test was not fully understood. In case of orally disintegrating tablet
H Sugimoto et al.
Current medicinal chemistry, 7(3), 303-339 (2000-01-19)
A wide range of evidence shows that acetylcholinesterase (AChE) inhibitors can interfere with the progression of Alzheimer's disease (AD). The successful development of these compounds was based on a well-accepted theory that the decline in cognitive and mental functions associated
Koteswara Rao Valasani et al.
Journal of chemical information and modeling, 53(8), 2033-2046 (2013-06-20)
Acetylcholinesterase (AChE) is a main drug target, and its inhibitors have demonstrated functionality in the symptomatic treatment of Alzheimer's disease (AD). In this study, a series of novel AChE inhibitors were designed and their inhibitory activity was evaluated with 2D
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.