추천 제품
Grade
pharmaceutical primary standard
API family
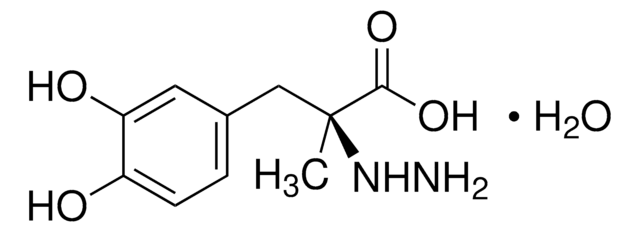
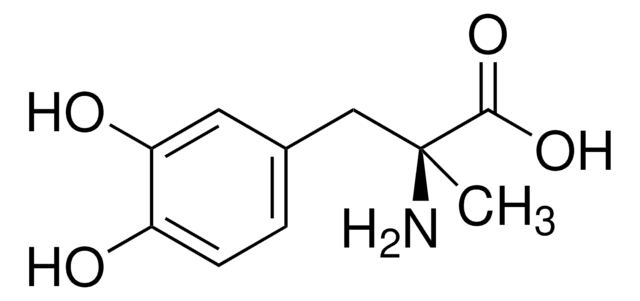
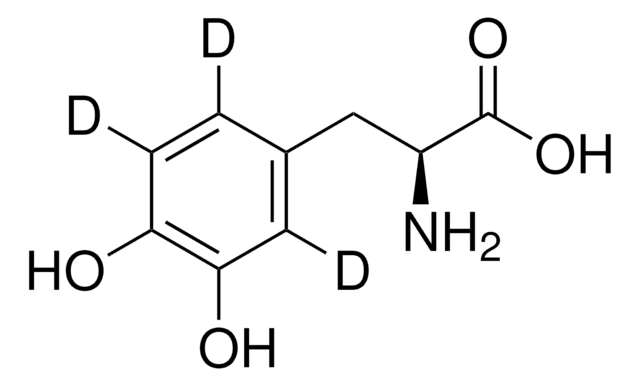
levodopa
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
InChI
1S/C10H13NO4/c1-15-9-5-6(2-3-8(9)12)4-7(11)10(13)14/h2-3,5,7,12H,4,11H2,1H3,(H,13,14)
InChI key
PFDUUKDQEHURQC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Levodopa Related Compound B USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
Also, for use with USP monographs such as:
Also, for use with USP monographs such as:
- Carbidopa and Levodopa Orally Disintegrating Tablets
- Levodopa
- Carbidopa and Levodopa Tablets
- Carbidopa and Levodopa Extended-Release Tablets
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Thomas Müller et al.
Clinical neuropharmacology, 36(2), 52-54 (2013-03-19)
Levodopa (LD)/dopa decarboxylase inhibitor application increases 3-O-methyldopa (3-OMD) concentrations in association with methyl group transfers, which demand for the conversion of methionine to homocysteine. This accompanying reaction is partially reversible by methyl group-donating vitamins. The objective of this study was
Manuel Vaz-da-Silva et al.
Drugs in R&D, 9(6), 435-446 (2008-11-08)
Levodopa is the most effective symptomatic treatment for Parkinson's disease (PD), but its use is often associated with development of motor complications. These adverse responses to fluctuations in dopaminergic stimulation can be reduced by concomitant administration of a catechol-O-methyltransferase (COMT)
Christoph Saxer et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 802(2), 299-305 (2004-03-17)
A simple and rapid assay is described for the simultaneous analysis of levodopa (l-DOPA) and 3-O-methyldopa (3-OMD) in human plasma samples, applying an ion-pair reversed-phase liquid chromatographic method with electrochemical detection, designed for clinical trials performed to study the effect
Teresa Nunes et al.
Clinical therapeutics, 31(10), 2258-2271 (2009-11-20)
Nebicapone is a reversible catechol-O-methyltransferase (COMT) inhibitor. Coadministration of a COMT inhibitor with levodopa and a dopa-decarboxylase inhibitor (carbidopa or benserazide) increases levodopa exposure and its therapeutic effect. The primary objective of this study was to investigate the effect of
T Müller et al.
Journal of neural transmission (Vienna, Austria : 1996), 114(3), 347-350 (2006-08-26)
Levodopa (LD) application improves motor symptoms in patients with Parkinson's disease (PD) patients. Little is known on further effects of LD, which induced lower plasma levels of cortisol and lower serotonergic activity in certain brain areas of fish. Objectives of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.