All Photos(1)
About This Item
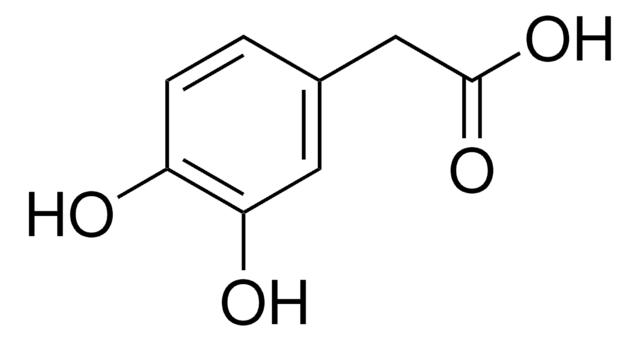
Linear Formula:
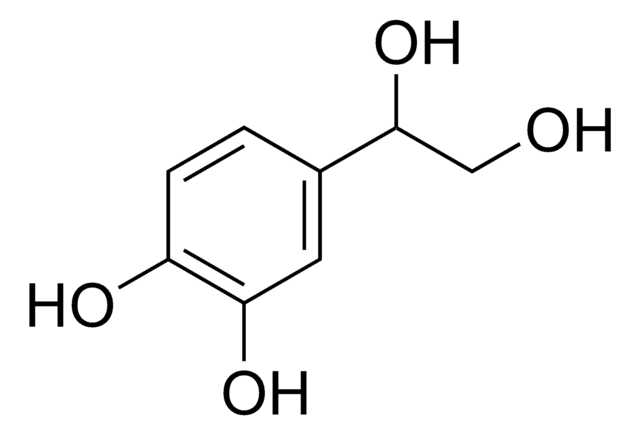
(HO)2C6H3CH(OH)CO2H
CAS Number:
Molecular Weight:
184.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
mp
136-137 °C (dec.) (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
OC(C(O)=O)c1ccc(O)c(O)c1
InChI
1S/C8H8O5/c9-5-2-1-4(3-6(5)10)7(11)8(12)13/h1-3,7,9-11H,(H,12,13)
Inchi Key
RGHMISIYKIHAJW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Metabolite of norepinephrine.
Application
DL-3,4-Dihydroxymandelic acid was used in the simultaneous analysis of 4-hydroxy-3-methoxymandelic acid and 4-hydroxy- 3-methoxyphenylacetic acid in urine. It was also used to study the changes in body temperature.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Andrea E Schwaninger et al.
Drug metabolism and disposition: the biological fate of chemicals, 39(11), 1998-2002 (2011-07-29)
Different pharmacokinetic properties are known for the two enantiomers of the entactogen 3,4-methylendioxy-methamphetamine (MDMA), most likely due to enantioselective metabolism. The aim of the present work was 1) the investigation of the main sulfotransferases (SULT) isoenzymes involved in the sulfation
T R Kingsley et al.
Journal of gerontology, 46(4), B135-B141 (1991-07-01)
Adrenal catecholamines (CA) were measured in 6-, 18-, and 30-mo Lobund-Wistar rats (LWR) maintained under germ-free or conventional conditions and fed either ad libitum or a restricted (70% of adult ad libitum) diet. Levels of dopamine (DA), norepinephrine (NE), epinephrine
Mechanistic studies on tyrosinase-catalysed oxidative decarboxylation of 3,4-dihydroxymandelic acid.
M Sugumaran et al.
The Biochemical journal, 281 ( Pt 2), 353-357 (1992-01-15)
Mushroom tyrosinase, which is known to convert a variety of o-diphenols into o-benzoquinones, has been shown to catalyse an unusual oxidative decarboxylation of 3,4-dihydroxymandelic acid to 3,4-dihydroxybenzaldehyde [Sugumaran (1986) Biochemistry 25, 4489-4492]. The mechanism of this reaction was re-investigated. Although
J N Rodríguez-López et al.
Analytical biochemistry, 195(2), 369-374 (1991-06-01)
A continuous spectrophotometric method for the rapid determination of diphenolase activity of tyrosinase is described. It uses 3,4-dihydroxymandelic acid (DOMA) as the substrate of tyrosinase and measures the final product, 3,4-dihydroxybenzaldehyde (DOBA). The spectrum of this product shows a bathochromic
K E O'Connor et al.
Journal of bacteriology, 183(3), 928-933 (2001-02-24)
Pseudomonas putida F6 was found to metabolize p-hydroxyphenylacetic acid through 3,4-dihydroxyphenylacetic acid, 3,4-dihydroxymandelic acid, and 3,4-dihydroxybenzaldehyde. Cell extracts of P. putida F6 catalyze the NAD(P)H-independent hydroxylation of p-hydroxyphenylacetic acid to 3,4-dihydroxyphenylacetic acid which is further oxidized to 3,4-dihydroxymandelic acid. Oxidation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service