153516
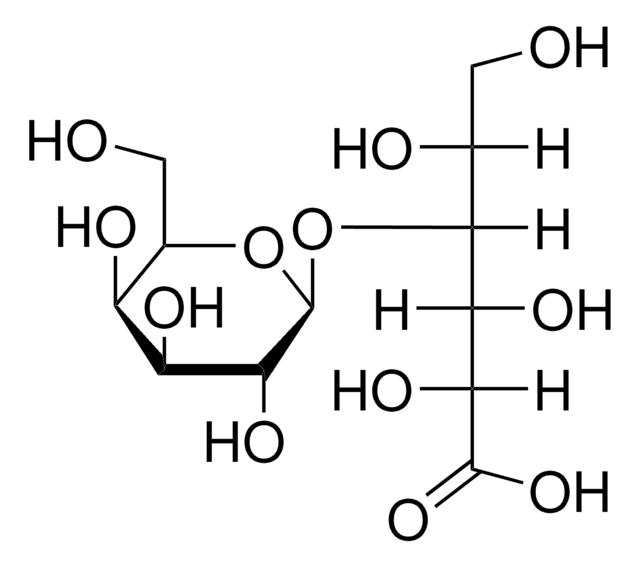
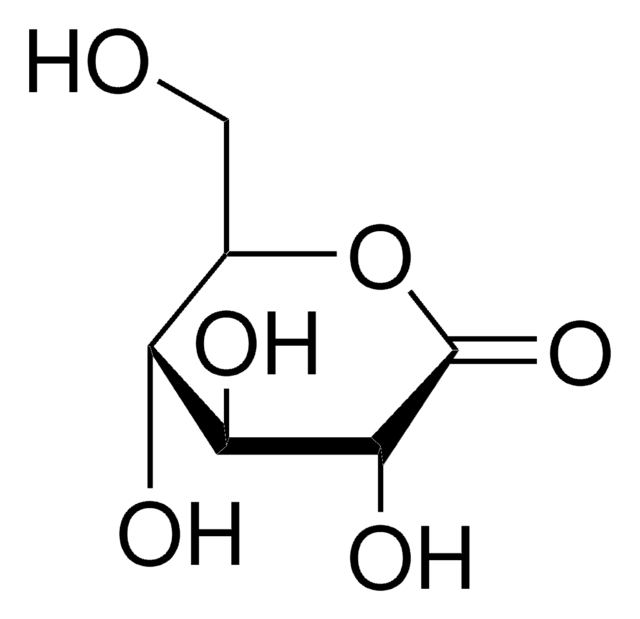
Lactobionic acid
97% (TLC)
Synonym(s):
4-O-β-D-Galactopyranosyl-D-gluconic acid
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C12H22O12
CAS Number:
Molecular Weight:
358.30
Beilstein/REAXYS Number:
95054
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic
Quality Level
assay
97% (TLC)
form
powder or crystals
optical activity
[α]20/D +25°, c = 10 in H2O
color
white
mp
113-118 °C (lit.)
solubility
5%, clear, colorless
storage temp.
room temp
SMILES string
OC[C@@H](O)[C@@H](O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O)[C@H](O)[C@@H](O)C(O)=O
Looking for similar products? Visit Product Comparison Guide
Application
Lactobionic acid, an α-hydroxyacid (AHA) with antioxidation activity, is used in the development of cosmeceuticals for skin. Lactobionic acid is used in the development of targeting drug delivery systems, such as microcapsules.
Other Notes
To gain a comprehensive understanding of our extensive range of Disaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Barbara A Green et al.
Clinics in dermatology, 27(5), 495-501 (2009-08-22)
The hydroxyacids are represented by the alpha-hydroxyacids, beta-hydroxyacids, polyhydroxy acids, and bionic acids. Together, these ingredients form a class of compounds with unparalleled benefits to the skin and unprecedented usage in the cosmeceutical market in cosmetic and therapeutic formulations alike.
Jing Zhang et al.
Acta biomaterialia, 7(4), 1665-1673 (2010-12-07)
This paper demonstrates a general approach for fabrication of lactobionic chitosan microcapsules using layer-by-layer assembly via click chemistry. Chitosan was selectively modified with either azide (CHI-Az) or alkyne (CHI-Alk) groups. The growth of the CHI-Az/CHI-Alk click multilayer was studied experimentally
Fang Wu et al.
Molecular pharmaceutics, 6(5), 1506-1517 (2009-07-30)
We aim to define the role of Kupffer cells in intrahepatic antigen presentation, using the selective delivery of antigen to Kupffer cells rather than other populations of liver antigen-presenting cells. To achieve this we developed a novel antigen delivery system
Saúl Alonso et al.
Bioresource technology, 109, 140-147 (2012-02-09)
The influence of dissolved oxygen availability on cell growth and lactobionic acid production from whey by Pseudomonas taetrolens has been investigated for the first time. Results from pH-shift bioreactor cultivations have shown that high agitation rate schemes stimulated cell growth
Chang-Moon Lee et al.
Magnetic resonance in medicine, 62(6), 1440-1446 (2009-10-28)
Hepatocyte-specific targeting agents are useful for evaluation of the hepatocytic function and the monitoring of disease progress. Superparamagnetic iron oxide nanoparticles (SPION) bearing terminal galactose groups exhibit a high affinity for the asialoglycoprotein receptor on the hepatocyte surface. In this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service