244112
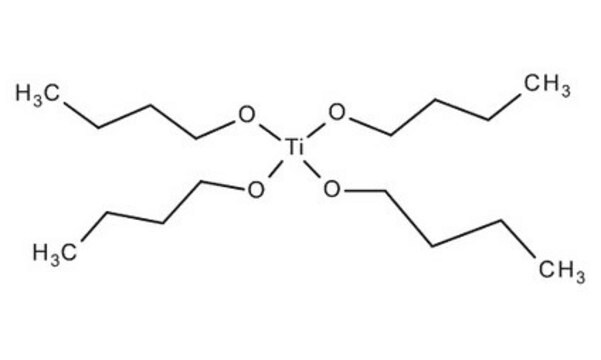
Titanium(IV) butoxide
reagent grade, 97%
Synonym(s):
Orthotitanic acid tetrabutylester, TNBT, TYZOR® TBT organic titanate, Tetrabutyl orthotitanate, Tetrabutyl titanate
About This Item
Recommended Products
grade
reagent grade
Quality Level
assay
97%
form
liquid (or viscous liquid)
liquid
reaction suitability
core: titanium
reagent type: catalyst
refractive index
n20/D 1.491 (lit.)
bp
206 °C/10 mmHg (lit.)
density
1.00 g/mL at 20 °C (lit.)
SMILES string
CCCCO[Ti](OCCCC)(OCCCC)OCCCC
InChI
1S/4C4H9O.Ti/c4*1-2-3-4-5;/h4*2-4H2,1H3;/q4*-1;+4
InChI key
YHWCPXVTRSHPNY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a catalyst in the transesterification of methyl methacrylate and synthesis of renewable polyesters from vanillin-based dimers.
- To synthesize ferroelectric Bi4Ti3O12 thin films.
- As a catalyst in the synthesis of aliphatic−aromatic copolyesters through polycondensation reactions.
- Titanium dioxide (TiO2) nanocomposite powders prepared via sol-gel synthesis used for photocatalytic degradation of pollutants.
- TiO₂ nanoparticles (anatase and rutile) via polyol-mediated synthesis technique.
- Titanium dioxide nanorods via hydrothermal method which are utilized as electron transport layers (ETLs) in perovskite solar cells (PSCs)
- TiO2 coatings enhance the stability and luminescent properties of Rb0.1Cs0.9PbBrI2 perovskite quantum dots (PQDs) for white-emitting diodes (WLEDs) and color conversion in display devices.
Features and Benefits
- TBO has a low viscosity, enables improved penetration and uniform coating on various substrates.
- It exhibits good thermal stability, which is advantageous during high-temperature processing, allowing it to maintain its chemical integrity and performance under elevated temperatures.
- It is soluble in anhydrous ethanol, ether, benzene and chloroform.
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Central nervous system, Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
107.6 °F - Pensky-Martens closed cup
flash_point_c
42 °C - Pensky-Martens closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Titanium dioxide applications: Semiconducting material characteristics and diverse functionalities.
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Protocols
Polymeric spheres serve as crystal templates. Synthesis methods yield large quantities.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service