364010
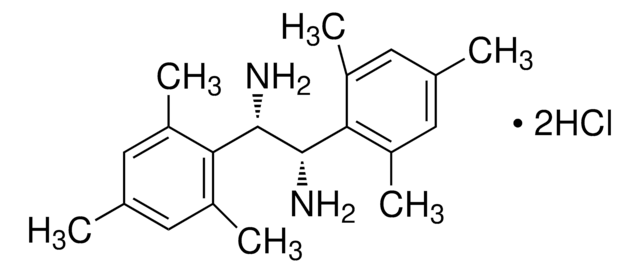
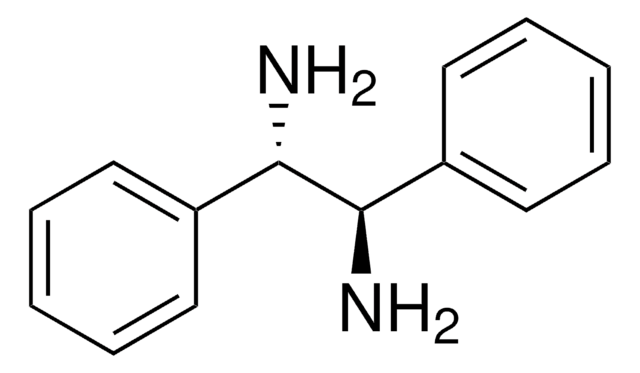
(1R,2R)-(+)-1,2-Diphenylethylenediamine
97%
Synonym(s):
(1R,2R)- DPEDA, (1R,2R)-DPEN, (1R,2R)-(+)-1,2-Diamino-1,2-diphenylethane
About This Item
Recommended Products
Quality Level
assay
97%
optical activity
[α]20/D +102°, c = 1 in ethanol
optical purity
ee: 99% (GLC)
mp
79-83 °C (lit.)
functional group
amine
phenyl
SMILES string
N[C@@H]([C@H](N)c1ccccc1)c2ccccc2
InChI
1S/C14H16N2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14H,15-16H2/t13-,14-/m1/s1
InChI key
PONXTPCRRASWKW-ZIAGYGMSSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- (1R,2R)-(+)-1,2-Diphenylethylenediamine (DPEN) is one of the widely used chiral auxiliary and ligand in the synthesis of asymmetric catalysts such as BINAP/diamine-Ru complexes for the stereoselective hydrogenation of ketones.
- It can be used in the preparation of monosulfonyl DPEN-salt to catalyze Michael addition of various ketones to maleimides.
- DPEN-derived chiral triazolium salts can be used to catalyze enantioselective intramolecular Stetter reaction and oxodiene Diels-Alder reaction.
- Zinc acetate complexes of DPEDA-derived ligands can also be used to catalyze hydrosilylation of imines.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![(1R,2R)-(+)-N,N′-Dimethyl-1,2-bis[3-(trifluoromethyl)phenyl]ethanediamine 97%](/deepweb/assets/sigmaaldrich/product/structures/408/938/05de1ba4-8e30-49a7-996e-99aa9340a1f4/640/05de1ba4-8e30-49a7-996e-99aa9340a1f4.png)