674613
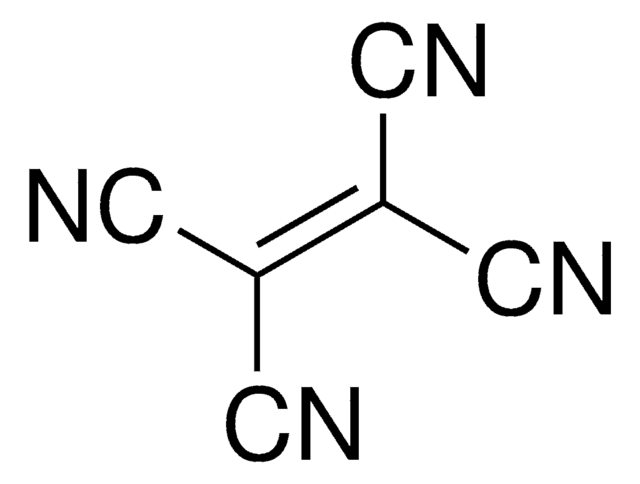
Tetrakis(dimethylamino)ethylene
Synonym(s):
Octamethylethylenetetramine, TDAE
About This Item
Recommended Products
Quality Level
reaction suitability
reaction type: C-C Bond Formation
refractive index
n20/D 1.48 (lit.)
bp
59 °C/0.9 mmHg (lit.)
density
0.861 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CN(C)\C(N(C)C)=C(\N(C)C)N(C)C
InChI
1S/C10H24N4/c1-11(2)9(12(3)4)10(13(5)6)14(7)8/h1-8H3
InChI key
CBXRMKZFYQISIV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Acts as a reducing agent
Reactant involved in:
- Dioxygenation
- Insertion of aluminum anion into the C-N bond
- Chemiluminescent reactions with oxygen
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
127.4 °F - closed cup
flash_point_c
53 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
In 2001, Professor William Dolbier, Jr., at the University of Florida reported1 an approach to nucleophilic trifluoromethylation based on the generation of a trifluoromethyl anion using CF3I in the presence of a powerful twoelectron reductant, tetrakis(dimethylamino)ethylene (TDAE)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)