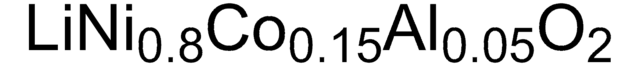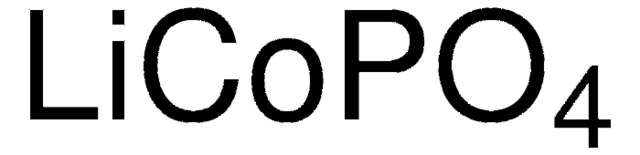757365
Lithium nickel dioxide
powder, <3 μm particle size (BET), ≥98% trace metals basis
Synonym(s):
LNO, Lithium nickel oxide, Lithium nickelate
About This Item
Recommended Products
grade
battery grade
assay
≥98% trace metals basis
form
powder
mol wt
Mw 97.63 g/mol
composition
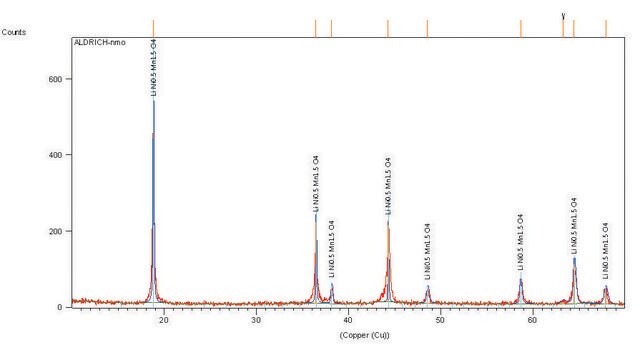
LiNiO2
particle size
<3 μm (BET)
mp
>1,000 °C (lit.)
density
4.62 g/cm3 (lit.)
application(s)
battery manufacturing
InChI
1S/Li.Ni.2O/q+1;;;-1
InChI key
VROAXDSNYPAOBJ-UHFFFAOYSA-N
Related Categories
General description
Application
Legal Information
related product
signalword
Danger
hcodes
Hazard Classifications
Carc. 1A Inhalation - Skin Sens. 1 - STOT RE 1
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Qiao's review explores stable microstructures for lithium metal fluoride batteries, advancing energy storage technologies.
Solid oxide fuel cells and electrolyzers show potential for chemical-to-electrical energy conversion, despite early development stages.
Li-ion batteries are currently the focus of numerous research efforts with applications designed to reduce carbon-based emissions and improve energy storage capabilities.
Lithium-ion batteries offer high energy density and cyclic performance for portable electronic devices.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service