W382108
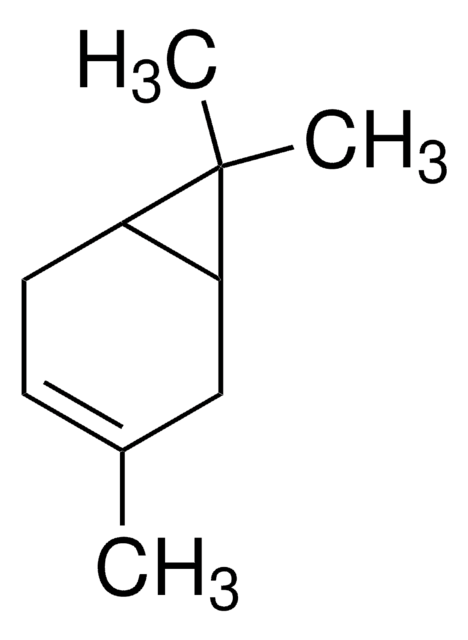
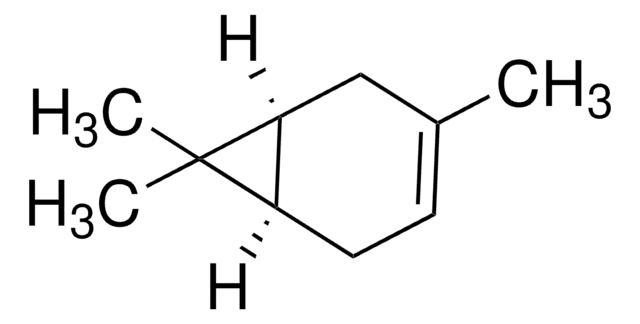
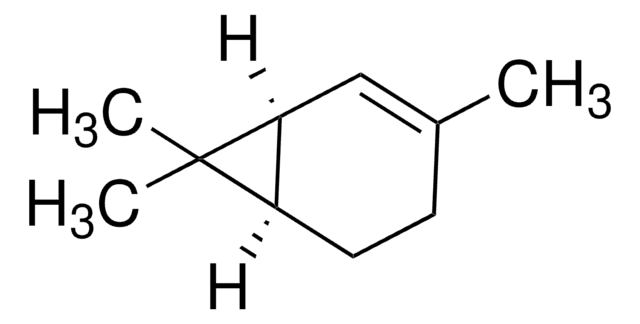
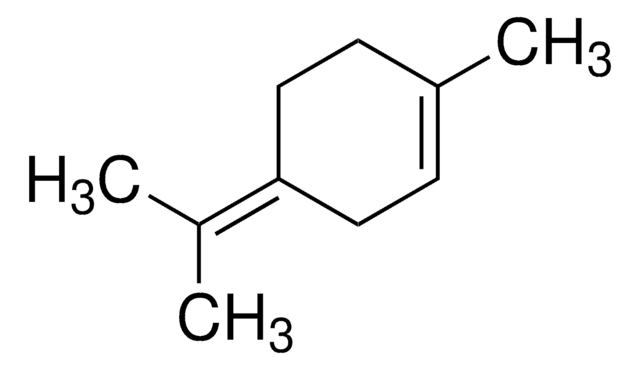
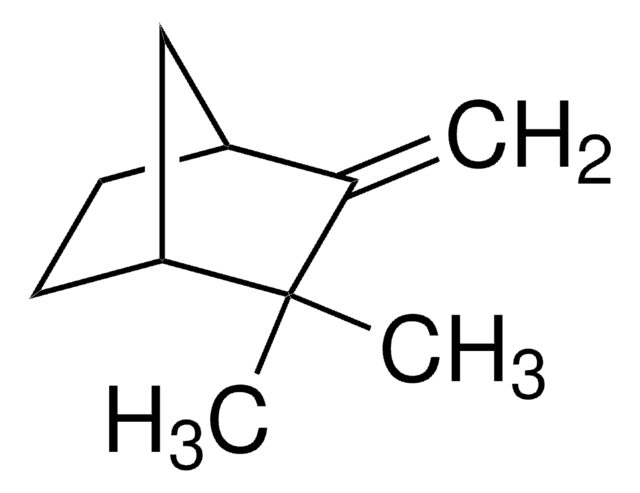
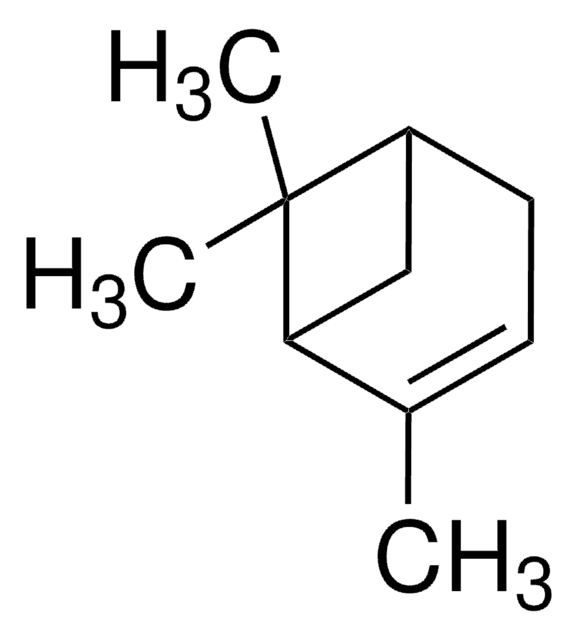
3-Carene
≥90%
Synonym(s):
δ3-Carene, 3,7,7-Trimethylbicyclo[4.1.0]hept-3-ene
About This Item
Recommended Products
biological source
synthetic
Quality Level
assay
≥90%
refractive index
n20/D 1.474 (lit.)
bp
168-169 °C/705 mmHg (lit.)
density
0.857 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
organoleptic
citrus; sweet
SMILES string
CC1=CCC2C(C1)C2(C)C
InChI
1S/C10H16/c1-7-4-5-8-9(6-7)10(8,2)3/h4,8-9H,5-6H2,1-3H3
InChI key
BQOFWKZOCNGFEC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Identification and quality evaluation of Lushan Yunwu tea from different geographical origins based on metabolomics.: The presence of 3-carene in Lushan Yunwu tea is studied for its impact on tea quality and flavor profile, providing insights into the metabolomic distinctions influenced by geographical factors (Sun et al., 2024).
- Influence of Maqian essential oil on gut microbiota and immunoresponses in type 1 diabetes: In silico study.: This in silico study investigates how 3-carene, as a component of Maqian essential oil, influences the gut microbiota and immune responses in type 1 diabetes, suggesting potential therapeutic uses of 3-carene in managing autoimmune diseases (Dahab et al., 2024).
Disclaimer
signalword
Danger
hcodes
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
116.6 °F - closed cup
flash_point_c
47 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-β-Farnesene; α-Huµlene; Germacrene D; (+)-Valencene; Bicyclogermacrene; (+)-δ-Cadinene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service