68069
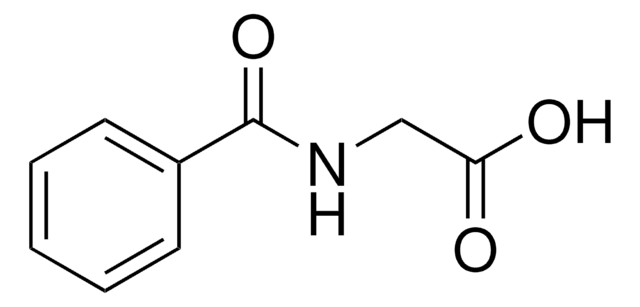
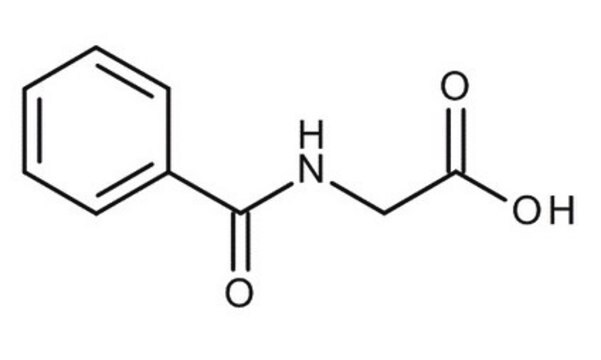
Hippuric acid
analytical standard
Synonym(s):
N-Benzoylglycine, Benzoylaminoacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CONHCH2COOH
CAS Number:
Molecular Weight:
179.17
Beilstein/REAXYS Number:
1073987
EC Number:
MDL number:
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
analytical standard
assay
≥97.5% (GC)
shelf life
limited shelf life, expiry date on the label
mp
187-191 °C (lit.)
application(s)
clinical testing
format
neat
SMILES string
OC(=O)CNC(=O)c1ccccc1
InChI
1S/C9H9NO3/c11-8(12)6-10-9(13)7-4-2-1-3-5-7/h1-5H,6H2,(H,10,13)(H,11,12)
InChI key
QIAFMBKCNZACKA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hippuric acid, the glycine conjugate of benzoic acid, is formed via a biotransformation process. From scientific literature, it is reported to be found as an excretory product in urine. Hippuric acid is also known to be catabolically synthesized from benzene-type aromatic compounds, believed to be originated from various factors such as environmental exposures, basic human liver metabolism of protein catabolism, cyclic sugar type-compounds, quinic acid, etc.
Application
Hippuric acid may be used as an analytical reference standard for the quantification of the analyte in the following:
- Human biological fluids using high-performance liquid chromatography (HPLC) with UV detection.
- Human and rat urine samples using gas chromatography–mass spectrometry (GC–MS).
- Human urine samples using GC equipped with a flame ionization detector.
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Determination of benzoic acid and hippuric acid in human plasma and urine by high-performance liquid chromatography
Kubota K, et al.
Journal of Chromatography. B, Biomedical Sciences and Applications, 425, 67-75 (1988)
Simultaneous determination of urinary hippuric acid, o-, m-and p-methylhippuric acids, mandelic acid and phenylglyoxylic acid for biomonitoring of volatile organic compounds by gas chromatography-mass spectrometry.
Ohashi Y, et al.
Analytica Chimica Acta, 566(2), 167-171 (2006)
Ronald W Pero
Current clinical pharmacology, 5(1), 67-73 (2009-11-07)
Hippuric acid has been a major human metabolite for years. However, there is no well-known documented health benefit associated with it except for excretion of environmental-toxic exposures of aromatic compounds such as toluene, or from dietary protein degradation and re-synthesis
P Kongtip et al.
Journal of chromatography. B, Biomedical sciences and applications, 751(1), 199-203 (2001-03-10)
A modified method for the simultaneous determination of hippuric acid (HA) and o-, m- and p-methylhippuric acids (o-, m- and p-MHAs) in urine is described. These metabolites were extracted, derivatized into their methyl ester derivatives and analyzed using a gas
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service