11009
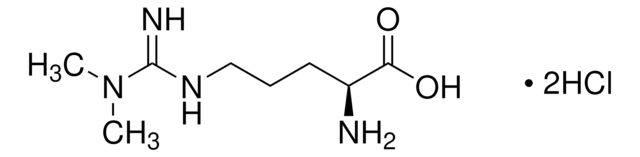
L-Arginine
BioUltra, ≥99.5% (NT)
Synonym(s):
(S)-2-Amino-5-guanidinopentanoic acid
About This Item
Recommended Products
product line
BioUltra
Quality Level
assay
≥99.5% (NT)
form
powder or crystals
optical activity
[α]20/D +27.0±0.5°, c = 5% in 5 M HCl
impurities
insoluble matter, passes filter test
≤0.3% foreign amino acids
ign. residue
≤0.05% (as SO4)
loss
≤0.1% loss on drying, 20 °C (HV)
color
white
pH
10.5-12.0 (25 °C, 0.5 M in H2O)
mp
222 °C (dec.) (lit.)
solubility
H2O: 0.5 M at 20 °C, clear, colorless
anion traces
chloride (Cl-): ≤100 mg/kg
sulfate (SO42-): ≤100 mg/kg
cation traces
Al: ≤5 mg/kg
As: ≤0.1 mg/kg
Ba: ≤5 mg/kg
Bi: ≤5 mg/kg
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
K: ≤50 mg/kg
Li: ≤5 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Mo: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Sr: ≤5 mg/kg
Zn: ≤5 mg/kg
λ
0.5 M in H2O
UV absorption
λ: 260 nm Amax: ≤0.2
λ: 280 nm Amax: ≤0.1
application(s)
peptide synthesis
SMILES string
N[C@@H](CCCNC(N)=N)C(O)=O
InChI
1S/C6H14N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h4H,1-3,7H2,(H,11,12)(H4,8,9,10)/t4-/m0/s1
InChI key
ODKSFYDXXFIFQN-BYPYZUCNSA-N
Gene Information
human ... NOS1(4842) , NOS2(4843)
rat ... Ppm1a(24666)
Looking for similar products? Visit Product Comparison Guide
Application
- L-Arginine-Dependent Nitric Oxide Production in the Blood of Patients with Type 2 Diabetes: A Pilot, Five-Year Prospective Study.: This study examines the role of L-Arginine in nitric oxide production in diabetic patients, proposing its potential benefits for managing vascular complications in diabetes (Stoian et al., 2024).
Biochem/physiol Actions
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service