67138
β-(1→3)-D-Glucanase from Helix pomatia
≥0.2 U/mg
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Helix pomatia
Quality Level
form
powder
specific activity
≥0.2 U/mg
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
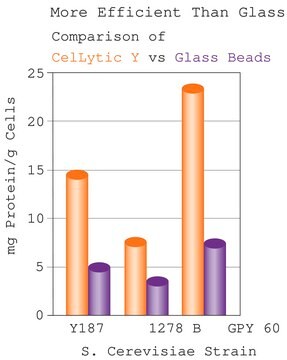
β-(1→3)-D-Glucanase from is used to digest β -1,3-glucan, which is a major component of cell walls. β-(1→3)-D-Glucanase from Helix pomatia has been used fto digest the cell walls of C. albicans .
Biochem/physiol Actions
Deletion of the C.albicans histidine kinase gene (CHK1) improves recognition by phagocytes through an increased exposure of cell wall b-1,3-glucans, which are readily digested by β-(1→3)-D-Glucanases .
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Unit Definition
One unit corresponds to the amount of enzyme which liberates 1 μmol of glucose from laminarin (Cat. No. 61340) per minute at pH 5.0 and 37 °C
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enrico Ragni et al.
Fungal genetics and biology : FG & B, 48(8), 793-805 (2011-05-24)
Cell wall biogenesis is a dynamic process relying on the coordinated activity of several extracellular enzymes. PHR1 is a pH-regulated gene of Candida albicans encoding a glycosylphosphatidylinositol-anchored β(1,3)-glucanosyltransferase of family GH72 which acts as a cell wall remodelling enzyme and
Alexander M Zakharenko et al.
Carbohydrate research, 346(2), 243-252 (2010-12-15)
The retaining endo-1,3-β-d-glucanase (EC 3.2.1.39) was isolated from the crystalline styles of the commercially available Vietnamese edible mussel Perna viridis. It catalyzes hydrolysis of β-1,3-bonds in glucans and enables to catalyze a transglycosylation reaction. Resources of mass-spectrometry for analysis of
Junio Cota et al.
Biochemical and biophysical research communications, 406(4), 590-594 (2011-03-01)
1,3-β-Glucan depolymerizing enzymes have considerable biotechnological applications including biofuel production, feedstock-chemicals and pharmaceuticals. Here we describe a comprehensive functional characterization and low-resolution structure of a hyperthermophilic laminarinase from Thermotoga petrophila (TpLam). We determine TpLam enzymatic mode of operation, which specifically
María de Medina-Redondo et al.
PloS one, 5(11), e14046-e14046 (2010-12-03)
The formation of the cell wall in Schizosaccharomyces pombe requires the coordinated activity of enzymes involved in the biosynthesis and modification of β-glucans. The β(1,3)-glucan synthase complex synthesizes linear β(1,3)-glucans, which remain unorganized until they are cross-linked to other β(1,3)-glucans
N P Sachivkina et al.
Bulletin of experimental biology and medicine, 149(6), 727-730 (2010-12-18)
Lyticase (a bacterial enzyme) was tested as a new antimycotic drug. Of all objects studied, Cellulomonas cellulans AC-870 strain proved to be most productive for this enzyme. A technology for lyticase isolation and purification was proposed. An experimental model of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service