G3907
Glutathione−Agarose
set of 3 pre-packed columns (2.5 ml each), (1:1 suspension in a 0.5 M NaCl + 20% ethanol solution)
Synonym(s):
GSH-agarose, S-linked glutathione agarose
About This Item
Recommended Products
Quality Level
form
(1:1 suspension in a 0.5 M NaCl + 20% ethanol solution)
analyte chemical class(es)
proteins (GST)
packaging
set of 3 pre-packed columns (2.5 ml each)
technique(s)
immunoprecipitation (IP): suitable
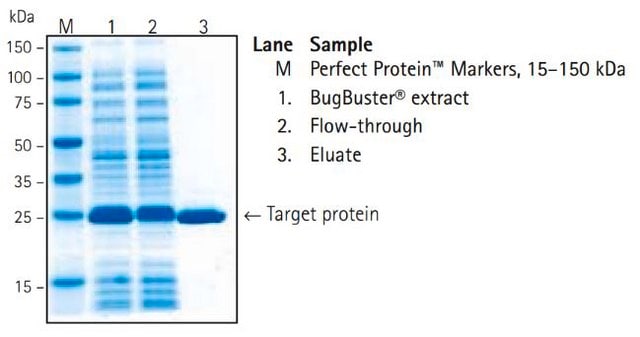
protein purification: suitable
matrix
cross-linked 4% beaded agarose
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
Application
The product was used in the design, synthesis and biological evaluation of new tryptamine and tetrahydro-β-carboline-based selective inhibitors of CDK4. It was also used to study Fascaplysin-inspired diindolyls as selective inhibitors of CDK4/cyclin D1.
Linkage
Physical form
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
180.1 °F
flash_point_c
82.3 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Investigate in vitro protein-protein interactions with pull-down assays, utilizing affinity, GST pull-down, TAP, and co-immunoprecipitation methods.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service