All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H21N3O5
CAS Number:
Molecular Weight:
275.30
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
γ-Glu-ε-Lys,
assay
≥98.0% (TLC)
Quality Level
form
powder
color
white
storage temp.
−20°C
SMILES string
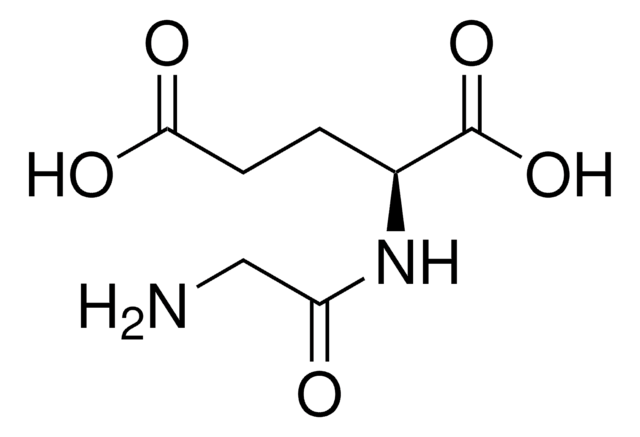
NC(CCCCNC(=O)CCC(N)C(O)=O)C(O)=O
InChI
1S/C11H21N3O5/c12-7(10(16)17)3-1-2-6-14-9(15)5-4-8(13)11(18)19/h7-8H,1-6,12-13H2,(H,14,15)(H,16,17)(H,18,19)
InChI key
JPKNLFVGUZRHOB-UHFFFAOYSA-N
Application
GammaGlu-epsilonLys (γ-Glu-ε-Lys) is used to study the functions and processing of endo-epsilon(-gamma-Glu)-Lys isopeptide bonds in biological processes.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L Zavalova et al.
Molecular & general genetics : MGG, 253(1-2), 20-25 (1996-11-27)
We previously detected in salivary gland secretions of the medicinal leech (Hirudo medicinalis) a novel enzymatic activity, endo-epsilon(gamma-Glu)-Lys isopeptidase, which cleaves isopeptide bonds formed by transglutaminase (Factor XIIIa) between glutamine gamma-carboxamide and the epsilon-amino group of lysine. Such isopeptide bonds
Damien M O Halloran et al.
Tissue engineering, 12(6), 1467-1474 (2006-07-19)
This study investigated the effect on the mechanical and physicochemical properties of type II collagen scaffolds after cross-linking with microbial transglutaminase (mTGase). It is intended to develop a collagen-based scaffold to be used for the treatment of degenerated intervertebral discs.
Thomas M Jeitner et al.
Amino acids, 44(1), 129-142 (2012-03-13)
Transglutaminases catalyze the formation of γ-glutamylamines utilizing glutamyl residues and amine-bearing compounds such as lysyl residues and polyamines. These γ-glutamylamines can be released from proteins by proteases in an intact form. The free γ-glutamylamines can be catabolized to 5-oxo-L-proline and
I P Baskova et al.
Biochemistry. Biokhimiia, 66(12), 1368-1373 (2002-01-29)
Destabilase, endo-epsilon-(gamma-Glu)-Lys-isopeptidase, was prepared from the salivary gland secretion of the medicinal leech (Hirudo medicinalis). The secretion prepared by the known method of Rigbi et al. (1987) (secretion-K) lacks the destabilase-characteristic highly specific isopeptidase activity (the D-dimer-monomerizing activity) because of
A Fradkov et al.
FEBS letters, 390(2), 145-148 (1996-07-22)
Earlier we detected a novel enzymatic activity in salivary gland secretion of the medicinal leech, splitting isopeptide bonds between the glutamine gamma-carboxamide and lysine epsilon-amino group. This activity is due to destabilase. We described its partial amino acid sequence and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service