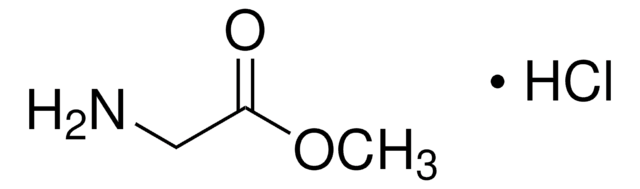
G6503
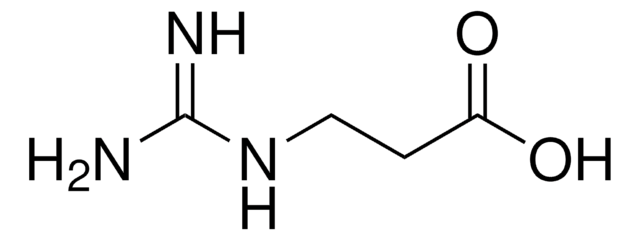
4-Guanidinobutyric acid
≥98%, for peptide synthesis
Synonym(s):
γ-Guanidinobutyric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH=C(NH2)NHCH2CH2CH2COOH
CAS Number:
Molecular Weight:
145.16
Beilstein/REAXYS Number:
1766447
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
4-Guanidinobutyric acid, ≥98%
Quality Level
assay
≥98%
form
powder or chunks
technique(s)
drug transporter assay: suitable
color
white
mp
280-282 °C
storage temp.
2-8°C
SMILES string
NC(=N)NCCCC(O)=O
InChI
1S/C5H11N3O2/c6-5(7)8-3-1-2-4(9)10/h1-3H2,(H,9,10)(H4,6,7,8)
InChI key
TUHVEAJXIMEOSA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Gamma-Guanidinobutyric acid (4-GBA) is used to study intestinal transport by the human proton-coupled amino acid transporter(s) (hPAT1). 4-GBA is used with other model compounds such as 1,6-diaminohexane, 1,6-diguanidinohexane, and 1,6-di(cyanoguanidino)hexane to study the biodegradability of the biocide polyhexamethylene biguanide (PHMB).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Al Banchaabouchi et al.
Metabolism: clinical and experimental, 47(3), 355-361 (1998-03-21)
Renal failure is characterized by the retention of nitrogenous metabolites such as urea, creatinine (CTN) and other guanidino compounds (GCs), uric acid, and hippuric acid, which could be related to the clinical syndrome associated with renal insufficiency. A model of
Disturbed metabolism of guanidino compounds characterized by elevated excretion of beta-guanidinopropionic acid and gamma-guanidinobutyric acid--an effect of vigabatrin treatment?
A Schulze et al.
Journal of inherited metabolic disease, 21(3), 268-271 (1998-08-01)
C Cabella et al.
European journal of biochemistry, 268(4), 940-947 (2001-02-17)
Rat hepatocytes in culture take up [14C]-agmatine by both a high-affinity transport system [KM = 0.03 mM; Vmax = 30 pmol x min x (mg protein)-1] and a low-affinity system. The high-affinity system also transports putrescine, but not cationic amino
Guanidino compounds in hyperargininemia.
B Marescau et al.
Advances in experimental medicine and biology, 153, 427-434 (1982-01-01)
Zhipan Zhang et al.
The journal of physical chemistry. B, 109(46), 21818-21824 (2006-07-21)
Dye-sensitized solar cells based on nanocrystalline TiO(2) have been fabricated with an amphiphilic ruthenium sensitizer [Ru (4,4'-dicarboxylic acid-2,2'-bipyridine) (4,4'-bis(p-hexyloxystyryl)-2,2'-bipyridine)(NCS)(2)], coded as K-19, and 4-guanidinobutyric acid (GBA) as coadsorbent. The cells showed a approximately 50 mV increase in open-circuit voltage and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service