Q2763
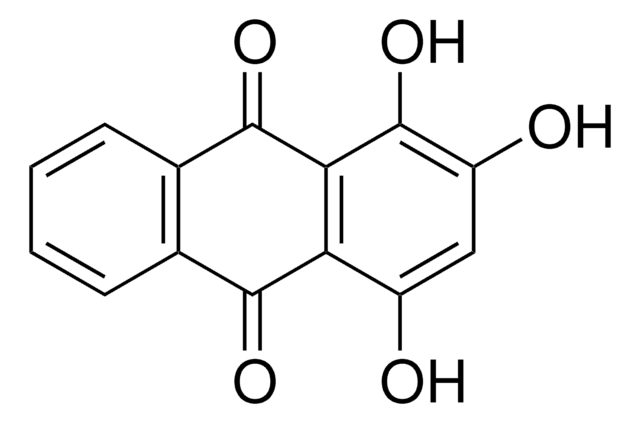
Quinalizarin
≥95% (HPLC), powder, plasmodium falciparum casein kinase 2α (PfCK2α) inhibitor
Synonym(s):
1,2,5,8-Tetrahydroxy-9,10-anthraquinone, 1,2,5,8-Tetrahydroxyanthraquinone, Alizarin Bordeaux BD, Alizarinbordeaux, Alizarine Bordeaux, Alizarine Bordeaux B, C.I. 58500, C.I. Mordant Violet 26, Khinalizarin, NSC 144046, NSC 4896, PHF 016
About This Item
Recommended Products
Product Name
Quinalizarin, ≥95% (HPLC)
assay
≥95% (HPLC)
form
powder
color
red
mp
≥300 °C
storage temp.
room temp
SMILES string
Oc1ccc2C(=O)c3c(O)ccc(O)c3C(=O)c2c1O
InChI
1S/C14H8O6/c15-6-3-4-7(16)11-10(6)12(18)5-1-2-8(17)13(19)9(5)14(11)20/h1-4,15-17,19H
InChI key
VBHKTXLEJZIDJF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- as a casein kinase II (CKII) inhibitor to study its effect on CKII-mediated phosphorylation of importin α on subcellular scaling in sperm chromosomes and egg extract[1]
- as a CKII inhibitor to study its ability to block parasite multiplication in a [3H]-hypoxanthine incorporation assay[2]
- to study its effect on colorectal cancer (CRC) cell cycle arrest, cell apoptosis, and reactive oxygen species (ROS) generation in SW480 and HCT-116 cell lines[3]
Biochem/physiol Actions
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service