1445547
USP
USP mAb 002, Monoclonal IgG1
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
Monoclonal IgG1
About This Item
Recommended Products
mol wt
~150,000 Da
packaging
pkg of 2 mg
manufacturer/tradename
USP
application(s)
pharmaceutical
format
neat
storage temp.
−70°C
General description
Can be used as:
- an independent control material for method development, training, and method transfer
- an internal assay control for standardization of physico-chemical testing
Application
- Size-Exclusion Chromatography
- Capillary SDS Electrophoresis (Reduced and Nonreduced)
- Analysis of N-Linked Oligosaccharides
- Sialic Acid Analysis
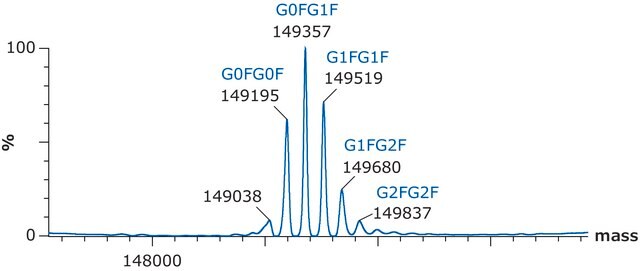
- Intact Mass
- Sequence identification by peptide mapping
Features and Benefits
- 4 Unique proteins with unique quality attributes
- Extensively characterized for confidence in method development
- Technical Note available
Analysis Note
Other Notes
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
Discover our wide variety of products for intact mass analysis of monoclonal antibodies, including size-exclusion columns (SEC), ion exchange columns, reverse-phase columns, HPLC buffers, MALDI matrices and standards, high-purity solvents, reagents, tools for protein sample preparation, and certified reference materials.
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service