おすすめの製品
品質水準
アッセイ
97%
形状
powder
mp
173-177 °C (lit.)
官能基
nitrile
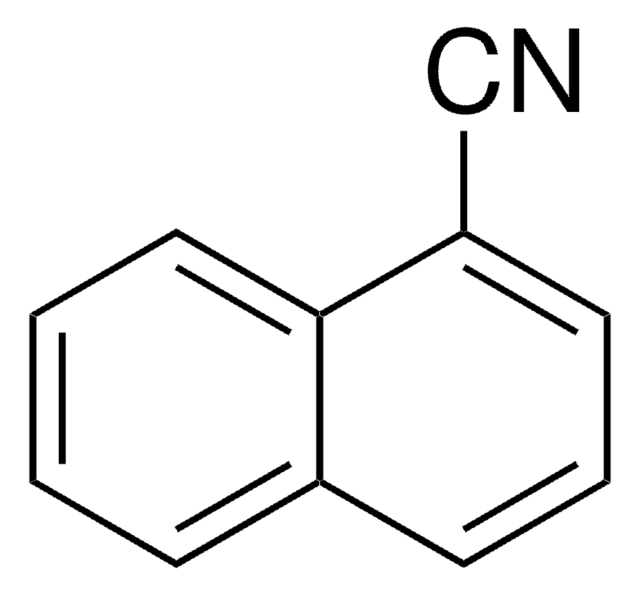
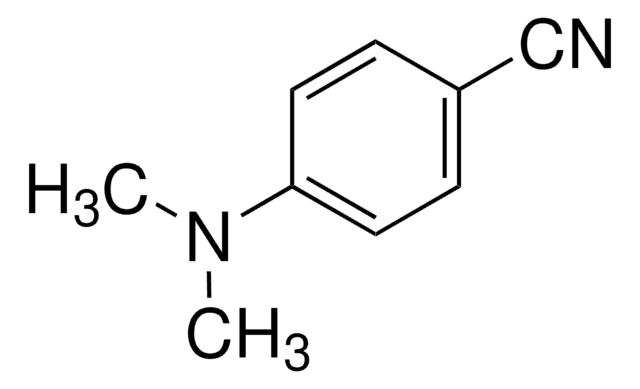
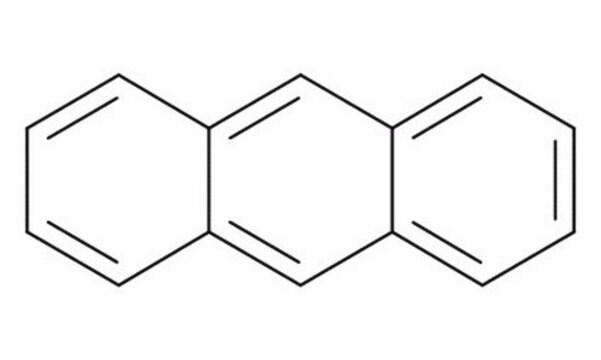
SMILES記法
N#Cc1c2ccccc2cc3ccccc13
InChI
1S/C15H9N/c16-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)15/h1-9H
InChI Key
KEQZHLAEKAVZLY-UHFFFAOYSA-N
詳細
The fluorescence excitation spectra of 9-anthracenecarbonitrile has been studied.
アプリケーション
9-Anthracenecarbonitrile was used to study the mechanism of charge separation within phenothiazine (PTZH) or phenoxazine (PXZH), and 9-cyanoanthracene(electron acceptor).
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
毒物及び劇物取締法
劇物
Jan Code
152765-5G:4548173106755
152765-25G:4548173106748
152765-VAR:
152765-BULK:
この製品を見ている人はこちらもチェック
O A Andreev et al.
Journal of muscle research and cell motility, 16(4), 353-367 (1995-08-01)
A serine residue located in the active site of myosin head (S1) was labelled by 9-anthroylnitrile, an amino group located in the central domain of S1 was labelled by 7-diethylamino-3-(4'-isothio-cyanato-phenyl)-4-methylcoumari n, a cysteine residue located near the C-terminus of S1
K Szarka et al.
Biochemistry, 40(49), 14806-14811 (2001-12-26)
It has been shown that one of the 12 serine residues within the 23 kDa segment of myosin subfragment 1 can be covalently modified with a fluorescent probe 9-anthroylnitrile (ANN) [Hiratsuka, T. (1989) J. Biol. Chem. 264 (30), 18188-18194]. To
Reika Kanya et al.
The Journal of chemical physics, 121(19), 9489-9497 (2004-11-13)
Fluorescence excitation spectra of the S(1)-S(0) origin band of 9-cyanoanthracene have been observed under a uniform electric field up to 200 kV/cm to explore pendular-state spectrum of an asymmetric-top molecule close to the strong field limit. The observed spectra exhibit
M Takahashi et al.
Journal of pharmaceutical and biomedical analysis, 14(11), 1579-1584 (1996-08-01)
9-Anthroylnitrile was used as an achiral reagent for the derivatization of carnitine. The reagent forms UV-absorbing derivatives with the hydroxyl groups of carnitine enantiomers under very mild conditions. The derivatives were separated by high-performance liquid chromatography on an ovomucoid-conjugated column
K H Kim et al.
Archives of pharmacal research, 21(6), 651-656 (1998-12-30)
A sensitive high-performance liquid chromatographic (HPLC) method for the determination of aloesin in plasma was developed. After solid-phase extraction from plasma and derivatization of aloesin and compound AD-1, which was prepared from aloesin as a internal standard, with 9-anthroylnitrile in
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)