おすすめの製品
品質水準
アッセイ
97%
形状
solid
bp
140 °C (lit.)
mp
37-39 °C (lit.)
官能基
fluoro
SMILES記法
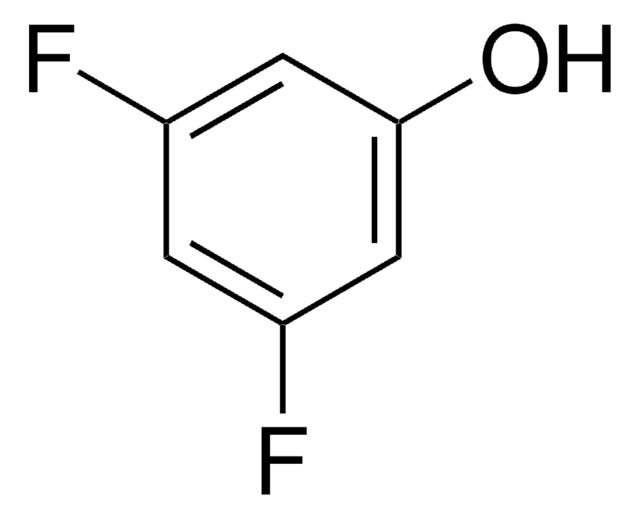
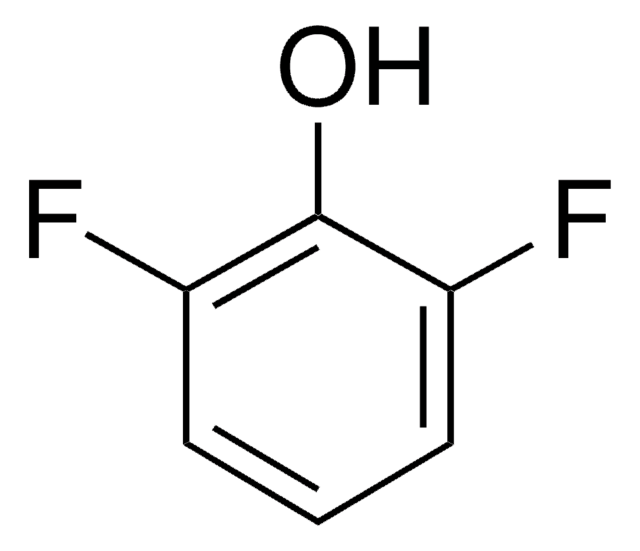
Oc1c(F)c(F)cc(F)c1F
InChI
1S/C6H2F4O/c7-2-1-3(8)5(10)6(11)4(2)9/h1,11H
InChI Key
PBYIIRLNRCVTMQ-UHFFFAOYSA-N
アプリケーション
2,3,5,6-テトラフルオロフェノールは以下のものの調製に使用されました。
- ペプチド合成で有用な放射性ヨウ素標識フェニルアラニン誘導体
- テクネチウム99m標識抗体
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
174.2 °F - closed cup
引火点(℃)
79 °C - closed cup
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
196789-5G:
196789-25G:
196789-BULK:
196789-100G:
196789-VAR:
この製品を見ている人はこちらもチェック
K Detmer et al.
The Journal of biological chemistry, 260(10), 5998-6005 (1985-05-25)
The oxidative half-reaction of phenol hydroxylase has been studied by stopped-flow spectrophotometry. Three flavin-oxygen intermediates can be detected when the substrate is thiophenol, or m-NH2, m-OH, m-CH3, m-Cl, or p-OH phenol. Intermediate I, the flavin C(4a)-hydroperoxide, has an absorbance maximum
A R Fritzberg et al.
Proceedings of the National Academy of Sciences of the United States of America, 85(11), 4025-4029 (1988-06-01)
Technetium-99m labeling of antibodies has been suboptimal because of low affinity adventitious binding, nonspecific labeling, and loss of immunoreactivity. The diamide dithiolate ligand system (N2S2) forms highly stable, well-defined tetradentate complexes with Tc(V). Antibodies and their fragments have been labeled
D S Wilbur et al.
Bioconjugate chemistry, 4(6), 574-580 (1993-11-01)
An investigation to prepare a phenylalanine derivative which could be radioiodinated and used directly in peptide synthesis was conducted. N-Boc-p-(tri-n-butylstannyl)-L-phenylalanine tetrafluorophenyl ester was targeted and synthesized from N-Boc-p-iodo-L-phenylalanine. The requisite aryl stannylation reaction was found to be best conducted using
C den Besten et al.
Chemical research in toxicology, 6(5), 674-680 (1993-09-01)
In the present study the oxidative dehalogenation of a para-halogenated phenol was studied using pentafluorophenol and its non-para-halogenated analogue 2,3,5,6-tetrafluorophenol as model compounds. 19F NMR was used to characterize the metabolite patterns. In order to study the primary oxidation products
G A Eiceman et al.
Journal of the American Society for Mass Spectrometry, 10(11), 1157-1165 (2001-09-07)
Atmospheric pressure chemical ionization (APCI)-mass spectrometry (MS) for fluorinated phenols (C6H5-xFxOH Where x = 0-5) in nitrogen with Cl- as the reagent ion yielded product ions of M Cl- through ion associations or (M-H)- through proton abstractions. Proton abstraction was
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)