おすすめの製品
アッセイ
≥95%
形状
powder
反応適合性
reaction type: solution phase peptide synthesis
利用可能性
available only in USA
アプリケーション
peptide synthesis
保管温度
2-8°C
アプリケーション
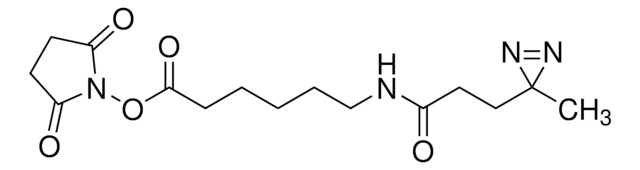
H-L-Photo-Methionine HCl is a diazirine-containing methionine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (~360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An Fmoc-protected version is also available as 907367.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
その他情報
Carboxyl-Photo-Reactive MS-Cleavable Cross-Linkers: Unveiling a Hidden Aspect of Diazirine-Based Reagents
A vimentin binding small molecule leads to mitotic disruption in mesenchymal cancer
Cell-Based Proteome Profiling of Potential Dasatinib Targets by Use of Affinity-Based Probes
Proteome profiling reveals potential cellular targets of staurosporine using a clickable cell-permeable probe
Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
A vimentin binding small molecule leads to mitotic disruption in mesenchymal cancer
Cell-Based Proteome Profiling of Potential Dasatinib Targets by Use of Affinity-Based Probes
Proteome profiling reveals potential cellular targets of staurosporine using a clickable cell-permeable probe
Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
関連製品
製品番号
詳細
価格
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Self-react. C
保管分類コード
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
907375-BULK:
907375-100MG:
907375-VAR:
最新バージョンのいずれかを選択してください:
Yeolin Lee et al.
Analytical chemistry, 88(19), 9503-9509 (2016-09-01)
Fc-specific antibody binding proteins (FcBPs) with the minimal domain of protein G are widely used for immobilization of well-oriented antibodies onto solid surfaces, but the noncovalently bound antibodies to FcBPs are unstable in sera containing large amounts of antibodies. Here
Claudio Iacobucci et al.
Analytical chemistry, 90(4), 2805-2809 (2018-01-30)
A major challenge in cross-linking/mass spectrometry (MS) is targeting carboxyl functions in proteins under physiological conditions that do not disturb the protein's conformation. Cross-linking of glutamic acid and aspartic acid residues in proteins will greatly expand the scope of structural
Haibin Shi et al.
Journal of the American Chemical Society, 134(6), 3001-3014 (2012-01-17)
Protein kinases (PKs) play an important role in the development and progression of cancer by regulating cell growth, survival, invasion, metastasis, and angiogenesis. Dasatinib (BMS-354825), a dual Src/Abl inhibitor, is a promising therapeutic agent with oral bioavailability. It has been
Michael J Bollong et al.
Proceedings of the National Academy of Sciences of the United States of America, 114(46), E9903-E9912 (2017-11-01)
Expression of the transcription factor FOXC2 is induced and necessary for successful epithelial-mesenchymal transition, a developmental program that when activated in cancer endows cells with metastatic potential and the properties of stem cells. As such, identifying agents that inhibit the
Xiaozhou Luo et al.
Proceedings of the National Academy of Sciences of the United States of America, 113(13), 3615-3620 (2016-03-16)
Thiopeptides are a subclass of ribosomally synthesized and posttranslationally modified peptides (RiPPs) with complex molecular architectures and an array of biological activities, including potent antimicrobial activity. Here we report the generation of thiopeptides containing noncanonical amino acids (ncAAs) by introducing
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)