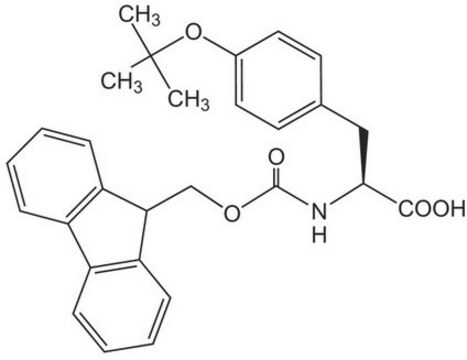
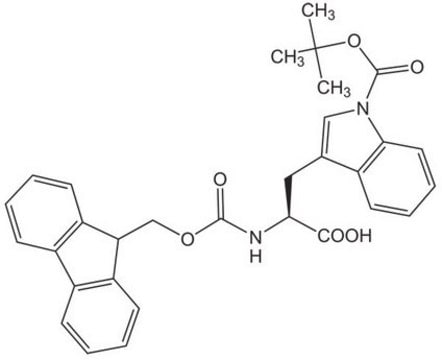
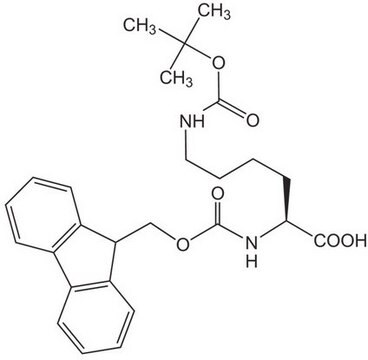
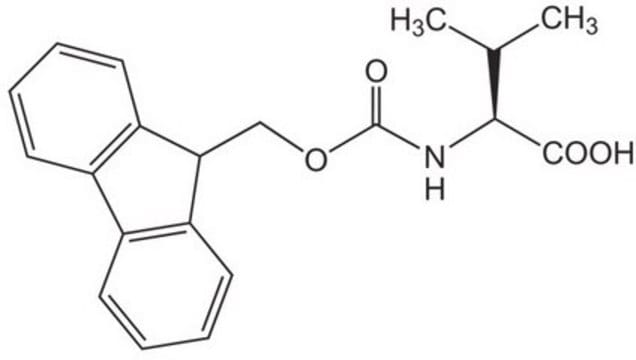
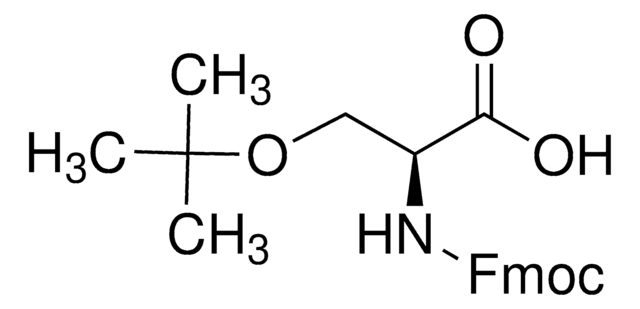
おすすめの製品
品質水準
製品種目
Novabiochem®
アッセイ
≥98% (TLC)
≥98.0% (acidimetric)
≥99.0% (HPLC)
形状
powder
反応適合性
reaction type: Fmoc solid-phase peptide synthesis
メーカー/製品名
Novabiochem®
mp
125-135 °C
アプリケーション
peptide synthesis
官能基
hydroxyl
保管温度
2-30°C
InChI
1S/C23H27NO5/c1-14(29-23(2,3)4)20(21(25)26)24-22(27)28-13-19-17-11-7-5-9-15(17)16-10-6-8-12-18(16)19/h5-12,14,19-20H,13H2,1-4H3,(H,24,27)(H,25,26)/p-1/t14-,20+/m1/s1
InChI Key
LZOLWEQBVPVDPR-VLIAUNLRSA-M
詳細
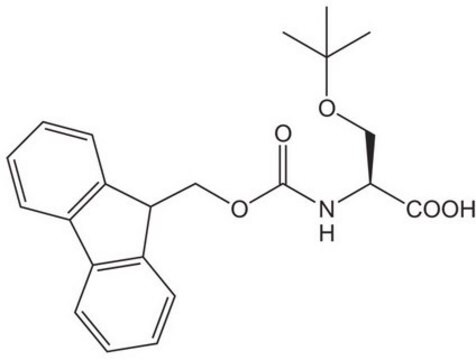
High purity Fmoc-protected amino acid for research and process production of peptides, with very low levels of dipeptide, free-amino acids and acetic acid impurities.
Standard building block for introduction of threonine amino-acid residues by Fmoc SPPS
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Standard building block for introduction of threonine amino-acid residues by Fmoc SPPS
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
アプリケーション
- Protocol for Facile Synthesis of Fmoc-N-Me-AA-OH Using 2-CTC Resin as Temporary and Reusable Protecting Group: This study demonstrates the effective use of Fmoc-Thr(tBu)-OH in the synthesis of N-methyl amino acids, which are crucial in developing peptidomimetic drugs (Román et al., 2023).
- Controlled Morphological Changes in Self-Assembled Structures Formed by Fmoc Variants of Threonine and Serine: Explores how Fmoc-Thr(tBu)-OH influences the self-assembly and structural morphology of peptidic materials, relevant in material science (Kshtriya et al., 2021).
- Solid-phase Synthesis of C-terminus Cysteine Peptide Acids: This article includes the use of Fmoc-Thr(tBu)-OH in the synthesis of peptides containing cysteine, which are significant in both drug design and biochemical studies (Mthembu et al., 2022).
- Polymer-Supported Stereoselective Synthesis of Benzoxazinothiadiazepinone 6,6-dioxides: Discusses the use of Fmoc-Thr(tBu)-OH in the stereoselective synthesis of novel organic compounds with potential applications in medicinal chemistry (Králová et al., 2017).
関連事項
Replaces: 04-12-1000
アナリシスノート
Colour (visual): white to off white
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.7 % (a/a)
Purity (HPLC): ≥ 99.0 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-ß-Ala-Thr(tBu)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Thr(tBu)-Thr(tBu)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Thr-OH (HPLC): ≤ 0.1 % (a/a)
Assay free amino acid (GC): ≤ 0.2 %
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Solubility (25 mmole in 50 ml DMF): clearly soluble
Assay (acidimetric): ≥ 98.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.02 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.7 % (a/a)
Purity (HPLC): ≥ 99.0 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-ß-Ala-Thr(tBu)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Thr(tBu)-Thr(tBu)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Thr-OH (HPLC): ≤ 0.1 % (a/a)
Assay free amino acid (GC): ≤ 0.2 %
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Solubility (25 mmole in 50 ml DMF): clearly soluble
Assay (acidimetric): ≥ 98.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.02 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
法的情報
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
適切な製品が見つかりませんか。
製品選択ツール.をお試しください
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
Yun Song et al.
Cell reports, 30(8), 2699-2711 (2020-02-27)
The transcriptional corepressor complex CoREST is one of seven histone deacetylase complexes that regulate the genome through controlling chromatin acetylation. The CoREST complex is unique in containing both histone demethylase and deacetylase enzymes, LSD1 and HDAC1, held together by the
関連コンテンツ
Purer Fmocs Means Purer Peptides
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)