おすすめの製品
product name
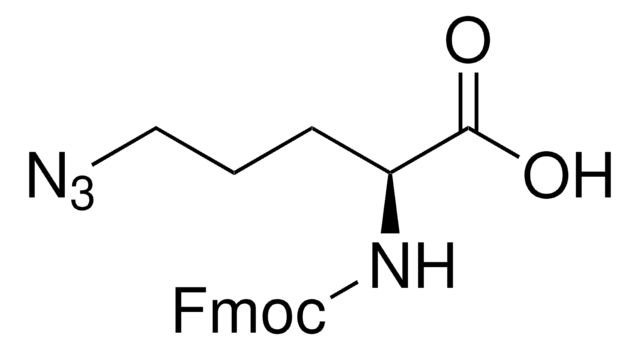
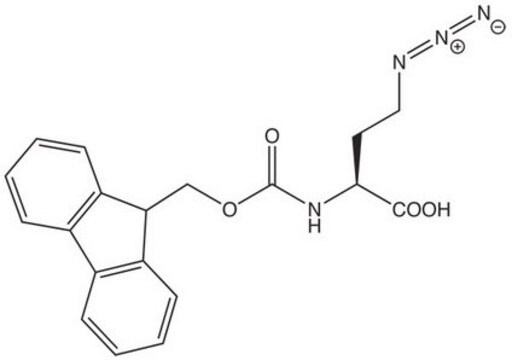
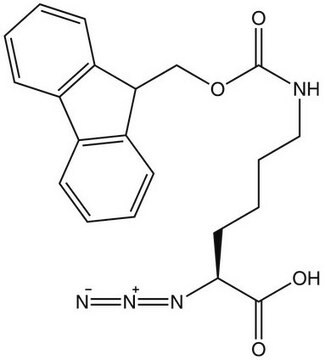
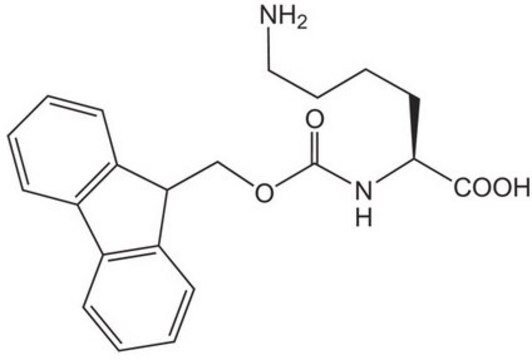
Fmoc-azidolysine, Novabiochem®
品質水準
製品種目
Novabiochem®
アッセイ
≥98.0% (HPLC)
形状
powder
反応適合性
reaction type: Fmoc solid-phase peptide synthesis
メーカー/製品名
Novabiochem®
アプリケーション
peptide synthesis
官能基
azide
保管温度
15-25°C
InChI
1S/C21H22N4O4/c22-25-23-12-6-5-11-19(20(26)27)24-21(28)29-13-18-16-9-3-1-7-14(16)15-8-2-4-10-17(15)18/h1-4,7-10,18-19H,5-6,11-13H2,(H,24,28)(H,26,27)/t19-/m0/s1
InChI Key
PJRFTUILPGJJIO-IBGZPJMESA-N
関連するカテゴリー
詳細
A useful tool for the synthesis of branched, side-chain modified and cyclic peptides and peptide tools for click chemistry by Fmoc SPPS. The side-chain azido group is completely stable to piperidine and TFA, but can be readily converted to an amine on the solid phase or in solution by reduction with thiols or phosphines. ,
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Guide to Selection of Orthogonally-Protected Building Blocks for Fmoc SPPS
Literature references:
[1] M. Meldal, et al. (1997) Tetrahedron Lett., 38, 2531.
[2] J. T. Lundquist & J. C. Pelletier (2001) Org. Lett., 3, 781.
[3] N. Nepomniaschiy, et al. (2008) Org. Lett., 10, 5243.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Guide to Selection of Orthogonally-Protected Building Blocks for Fmoc SPPS
Literature references:
[1] M. Meldal, et al. (1997) Tetrahedron Lett., 38, 2531.
[2] J. T. Lundquist & J. C. Pelletier (2001) Org. Lett., 3, 781.
[3] N. Nepomniaschiy, et al. (2008) Org. Lett., 10, 5243.
アプリケーション
Recent applications of Fmoc-azidolysine include:
- In the synthesis of a cationic cell-penetrating peptide (CPP) by click-chemistry.
- In the preparation of a peptide-resorcinarene conjugate by click chemistry.
アナリシスノート
Colour (visual): white to slight yellow to beige
Appearance of substance (visual): powder
Identity (IR): passes test
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Appearance of substance (visual): powder
Identity (IR): passes test
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
法的情報
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
Improved solid-phase peptide synthesis method utilizing alpha-azide-protected amino acids
J. T. Lundquist & J. C. Pelletier
Organic Letters, 3, 781-781 (2001)
Peptide-Resorcinarene Conjugates Obtained via Click Chemistry: Synthesis and Antimicrobial Activity
Pineda-Castaneda H, et al.
Antibiotics, 12, 773-773 (2023)
Cyclization of a cell-penetrating peptide via click-chemistry increases proteolytic resistance and improves drug delivery
Reichart F, et al.
Journal of Peptide Science, 22, 421-426 (2016)
Switch peptide via Staudinger reaction
N. Nepomniaschiy, et al.
Organic Letters, 10, 5243-5243 (2008)
Azido Acids in a Novel Method of Solid-Phase Peptide Synthesis.
M. Meldal, et al.
Tetrahedron Letters, 38, 2531-2531 (1997)
資料
Novabiochem® offers orthogonally protected amino acids for peptide synthesis, including cyclic and branched peptides.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)