おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to BP 462
traceable to Ph. Eur. N0750000
traceable to USP 1463508
APIファミリー
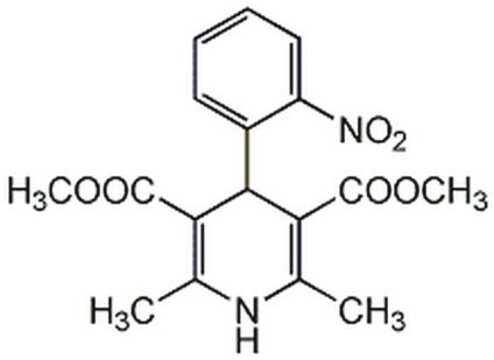
nifedipine
CofA
current certificate can be downloaded
テクニック
HPLC: suitable
gas chromatography (GC): suitable
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-30°C
SMILES記法
COC(=O)C1=C(C)NC(C)=C(C1c2ccccc2[N+]([O-])=O)C(=O)OC
InChI
1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3
InChI Key
HYIMSNHJOBLJNT-UHFFFAOYSA-N
遺伝子情報
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Nifedipine belongs to the class of calcium channel antagonist drugs and finds a wide use as an antihypertensive and an antianginal agent. It can also be used as a coronary vasodilator.
アプリケーション
アナリシスノート
その他情報
脚注
関連製品
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1290-1G-PW:
PHR1290-1G:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)