すべての画像(1)
About This Item
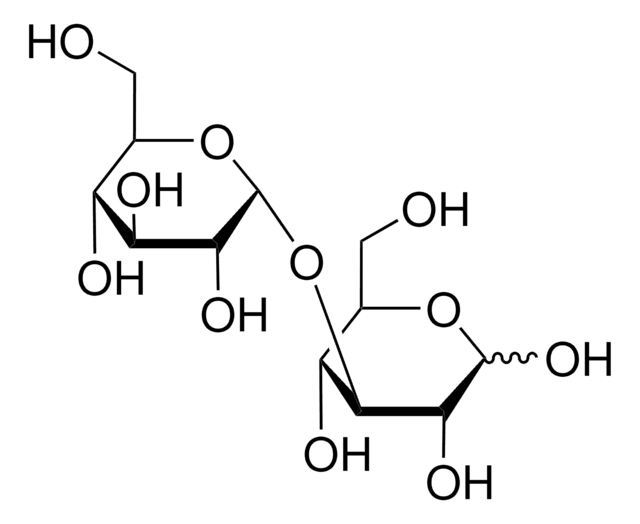
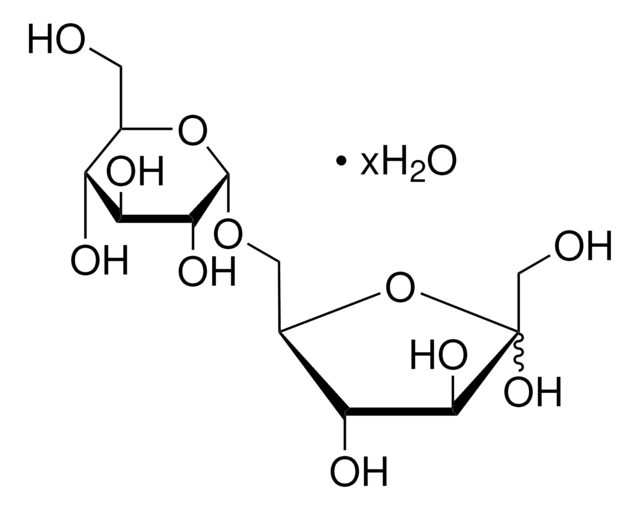
実験式(ヒル表記法):
C12H22O11
CAS番号:
分子量:
342.30
MDL番号:
UNSPSCコード:
12352201
PubChem Substance ID:
NACRES:
NA.25
おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
フォーム
powder
テクニック
HPLC: suitable
色
white to off-white
溶解性
water: 5 mg/mL, clear, colorless
保管温度
−20°C
SMILES記法
OCC(O)C(O)C(O)C(OC1OC(CO)C(O)C(O)C1O)C=O
InChI
1S/C12H22O11/c13-1-4(16)7(17)8(18)5(2-14)22-12-11(21)10(20)9(19)6(3-15)23-12/h2,4-13,15-21H,1,3H2
InChI Key
PZDOWFGHCNHPQD-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
Kojibiose, a disaccharide product of glucose caramelization and an inhibitor of plant glucosidase I, may be used to help identify and characterize glucosidase I enzymes involved in terminal deglycosylation of high-mannose oligosaccharides. Kojibiose may be used as a substrate to study the biological species, enzymes and catabolic processes that catabolize it as an energy source. Kojibiose may be used to identify, differentiate and characterize kojibiose phosphorylase(s) (KP).
生物化学的/生理学的作用
Kojibiose is an inhibitor of plant glucosidase I. It inhibits the removal of terminal glucose from the high-mannose oligosaccharide (Glc)3(Man)9(GlcNAc)2, either from the free oligosaccharide or from the oligosaccharide attached to a protein via N-linkage.
その他情報
To gain a comprehensive understanding of our extensive range of Disaccharides for your research, we encourage you to visit our Carbohydrates Category page.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
K4769-BULK:
K4769-VAR:
K4769-1MG:
この製品を見ている人はこちらもチェック
Satoshi Okada et al.
The FEBS journal, 281(3), 778-786 (2013-11-22)
Glycoside hydrolase (GH) family 65 contains phosphorylases acting on maltose (Glc-α1,4-Glc), kojibiose (Glc-α1,2-Glc), trehalose (Glc-α1,α1,-Glc), and nigerose (Glc-α1,3-Glc). These phosphorylases can efficiently catalyze the reverse reactions with high specificities, and thus can be applied to the practical synthesis of α-glucosyl
S Ogawa et al.
Carbohydrate research, 307(1-2), 83-95 (1998-07-11)
Two kojibiose-type pseudo-disaccharides and a trisaccharide, containing a 5-amino-1,2,3,4-cyclopentanetetrol derivative or valienamine, linked by way of nitrogen bridges to the sugar residues, have been designed and synthesized as processing alpha-glucosidase I inhibitors. Synthesis of the pseudo-disaccharides was carried out starting
Synthesis of linear oligosaccharides: L-glycero-alpha-D-manno-heptopyranosyl derivatives of allyl alpha-glycosides of D-glucose, kojibiose, and 3-O-alpha-kojibiosyl-D-glucose, substrates for synthetic antigens.
S A Nepogod'ev et al.
Carbohydrate research, 254, 43-60 (1994-02-17)
Ana Vila Verde et al.
The journal of physical chemistry. B, 115(21), 7069-7084 (2011-05-13)
Molecular level insight into water structure and structural dynamics near proteins, lipids, and nucleic acids is critical to the quantitative understanding of many biophysical processes. Unfortunately, understanding hydration and hydration dynamics around such large molecules is challenging because of the
Jarred Yasuhara-Bell et al.
Microorganisms, 8(3) (2020-03-11)
Rathayibacter toxicus is a Gram-positive, nematode-vectored bacterium that infects several grass species in the family Poaceae. Unique in its genus, R. toxicus has the smallest genome, possesses a complete CRISPR-Cas system, a vancomycin-resistance cassette, produces tunicamycin, a corynetoxin responsible for
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)