おすすめの製品
品質水準
アッセイ
≥97% (HPLC)
フォーム
powder
色
white to beige
溶解性
DMSO: 10 mg/mL, clear
保管温度
−20°C
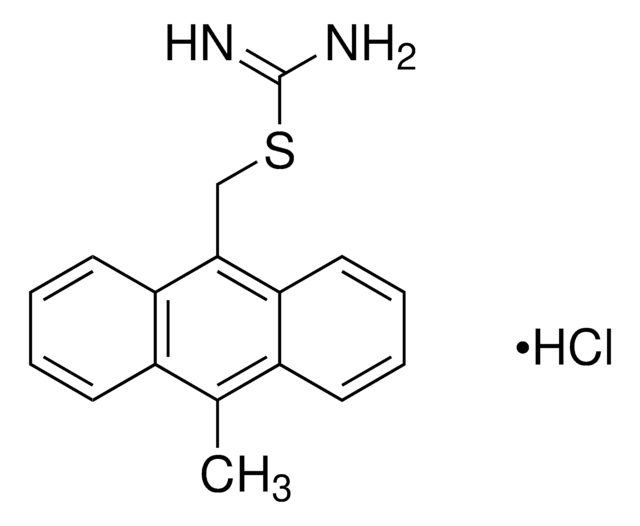
SMILES記法
S(C)c1[c](cc2c(cc1)c3c(cc(c(c3OC)OC)OC)CC[C@@H]2NC(=O)C)=O
InChI
1S/C22H25NO5S/c1-12(24)23-16-8-6-13-10-18(26-2)21(27-3)22(28-4)20(13)14-7-9-19(29-5)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1
InChI Key
CMEGANPVAXDBPL-INIZCTEOSA-N
生物化学的/生理学的作用
Thiocolchicine is an antimitotic alkaloid and apoptosis inducer that inhibits tubulin polymerization and microtubule assembly.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Eye Dam. 1 - Muta. 1B
保管分類コード
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML0954-25MG:
SML0954-VAR:
SML0954-BULK:
SML0954-5MG:
最新バージョンのいずれかを選択してください:
Dorota Bartusik et al.
Bioorganic chemistry, 38(1), 1-6 (2009-12-01)
It was shown, that cultured ex vivo human T-Lymphoblastoid (CEM) cells respond to synthesized thiocolchicine and fluorine thiocolchicine derivatives. The preparation of derivatives with substitution at C-3 and C-7 is described. All compounds were used at concentration from 1 nM
R M Chabin et al.
Biochemical and biophysical research communications, 161(2), 544-550 (1989-06-15)
Thiocolchicine, a colchicine analog in which the C-10 methoxy is replaced with a thiomethyl moiety, was shown to bind with high affinity to the colchicine site on tubulin (Ka = 1.07 +/- 0.14 x 10(6) M-1 at 23 degrees C).
A Muzaffar et al.
Journal of medicinal chemistry, 33(2), 567-571 (1990-02-01)
Esterification of the phenolic group in 3-demethylthiocolchicine and exchange of the N-acetyl group with other N-acyl groups or a N-carbalkoxy group afforded many compounds which showed superior activity over the parent drug as inhibitors of tubulin polymerization and of the
Bruno Danieli et al.
The Journal of organic chemistry, 71(7), 2848-2853 (2006-03-25)
A dynamic combinatorial library of thiocolchicine-podophyllotoxin derivatives based on the disulfide bond exchange reaction is described. The influence of a biological target on the composition of the reaction mixture has been demonstrated. Use of high-resolution ESI mass spectrometry to evaluate
R De Vincenzo et al.
Oncology research, 11(3), 145-152 (1999-10-20)
Three new 7-0-substituted deacetamidothiocolchicine derivatives have been evaluated for their antitumor activity against various human tumor cell lines, some of which express the multidrug resistance (MDR) phenotype, for their impact on the cell cycle and their binding to tubulin. Colchicine
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)