おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
carbidopa
メーカー/製品名
USP
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
SMILES記法
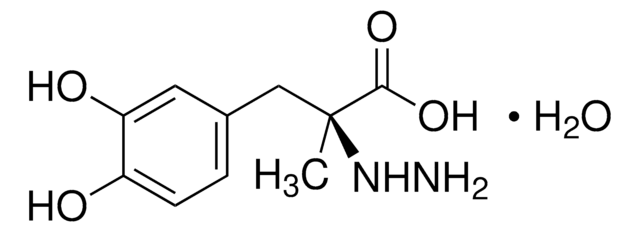
O.C[C@@](Cc1ccc(O)c(O)c1)(NN)C(O)=O
InChI
1S/C10H14N2O4.H2O/c1-10(12-11,9(15)16)5-6-2-3-7(13)8(14)4-6;/h2-4,12-14H,5,11H2,1H3,(H,15,16);1H2/t10-;/m0./s1
InChI Key
QTAOMKOIBXZKND-PPHPATTJSA-N
遺伝子情報
human ... DDC(1644)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Carbidopa USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Carbidopa and Levodopa Extended-Release Tablets
- Carbidopa and Levodopa Orally Disintegrating Tablets
- Carbidopa and Levodopa Tablets
アナリシスノート
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Eye Irrit. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 3
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
1095506-400MG:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
Mohseni Mehran S M et al.
Journal of clinical and diagnostic research : JCDR, 7(6), 1004-1007 (2013-08-02)
According to many studies, sprouted fava beans are a rich source of levo-dihydroxy phenylalanine (L-dopa) the precursor of dopamine, and they are now being investigated for use in the management of Parkinson's disease. The addition of Carbidopa (C-dopa) can reduce
Q. Alan Xu, Timothy L. Madden
Analytical Methods for Therapeutic Drug Monitoring and Toxicology, 80-80 (2011)
Ina Schabram et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 34(44), 14769-14776 (2014-10-31)
Methylphenidate (MPH) inhibits the reuptake of dopamine and noradrenaline. PET studies with MPH challenge show increased competition at postsynaptic D2/3-receptors, thus indirectly revealing presynaptic dopamine release. We used [(18)F]fluorodopamine ([(18)F]FDOPA)-PET in conjunction with the inlet-outlet model (IOM) of Kumakura et
J I Sage et al.
Clinical neuropharmacology, 17 Suppl 2, S1-S6 (1994-01-01)
This article reviews the pharmacokinetics of Sinemet CR, a controlled-release (CR) levodopa preparation. The main influences on the kinetic profile are as follows: absorption, which depends on the dissolution characteristics of the tablet, the pattern of gastric emptying, and the
J T Hutton et al.
Neurology, 42(1 Suppl 1), 51-56 (1992-01-01)
Parkinson's disease patients treated chronically with levodopa often develop fluctuations in motor response. Motor fluctuations can be attributed in part to oscillating plasma levodopa concentrations. A controlled-release formulation containing 200 mg of levodopa and 50 mg of carbidopa provides superior
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)