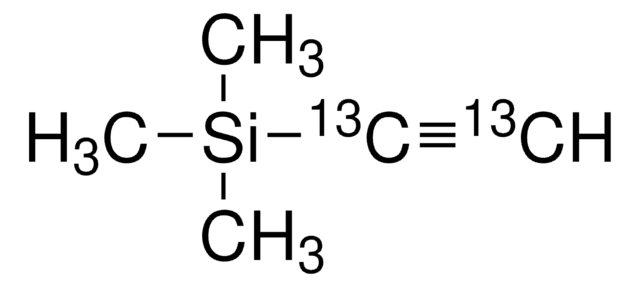추천 제품
양식
liquid
Quality Level
농도
0.5 M in THF
density
0.88 g/mL at 25 °C
SMILES string
[Li]C#C[Si](C)(C)C
InChI
1S/C5H9Si.Li/c1-5-6(2,3)4;/h2-4H3;
InChI key
ZVXXEONXFWSCIZ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Lithium (trimethylsilyl)acetylide can be used as a reagent:
- In the transmetalation and nucleophilic displacement reactions.
- To prepare propargylic alcohol derivatives by reacting with aldehydes and ketones.
- To synthesize α,βynones by treating with Weinreb amide or with isoxazolidide.
- Along with Grignard reagent to convert γ- and δ-thiolactams to thioiminium salts, which are employed as key intermediates to produce disubstituted pyrrolidines.
Reacts with halotriazines to produce conductive, anisotropic carbon-nitrogen materials.
포장
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
법적 정보
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
-9.4 °F - closed cup
Flash Point (°C)
-23 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Sequential addition reaction of lithium acetylides and Grignard reagents to thioiminium salts from thiolactams leading to 2, 2-disubstituted cyclic amines
M Toshiaki, et al.
Tetrahedron, 62(26), 6312-6320 (2006)
Lithium (Trimethylsilyl) acetylide
Fuhry MM
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2001)
Chemistry of Materials, 6, 636-636 (1994)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.