모든 사진(3)
About This Item
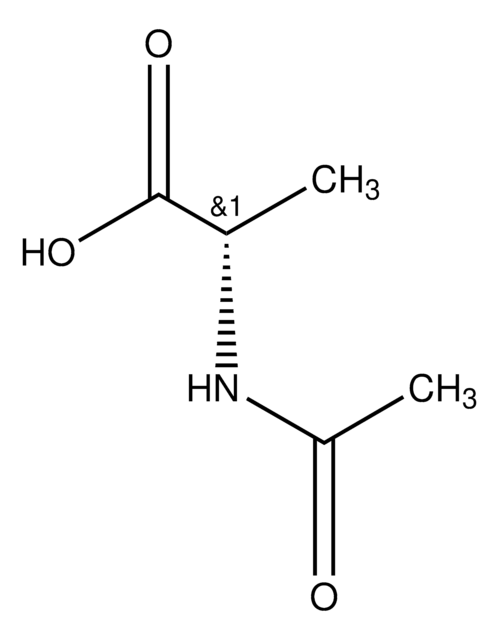
Linear Formula:
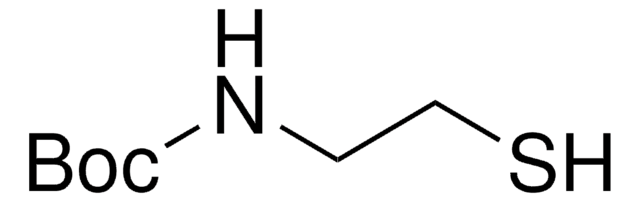
CH3CONHCH2CH2SH
CAS Number:
Molecular Weight:
119.19
MDL number:
UNSPSC 코드:
12352100
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
N-Acetylcysteamine, also known as N-(2-Mercaptoethyl) acetamide, is a derivative of cysteamine, that is commonly used as a building block for the synthesis of alkylated thiol and thioesters via esterification.
애플리케이션
N-Acetylcysteamine can be used as a building block to synthesize:
- N
- -acetylcysteamine (SNAC) thioesters by reacting with various acid derivatives in the presence of 1,1′-carbonyldiimidazole (CDI).
- Thieno[2,3-c]pyrrole derivatives via three-component reaction of 2-acetyl-3-thiophenecarboxaldehyde and various amines.
- Carbapenems, a class of beta-lactam antibiotic agents.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Cell cycle arrest and apoptosis induction by an anticancer chalcone epoxide
Haiyong H, et al
Archives of Pharmacal Research, 343, 429-439 (2010)
Isolation, properties, and regulation of a mitochondrial acyl coenzyme A thioesterase from pig heart.
K Y Lee et al.
The Journal of biological chemistry, 254(11), 4516-4523 (1979-06-10)
Magoichi Sako et al.
The Journal of organic chemistry, 63(20), 6947-6951 (2001-10-24)
4H-[1,2,5]Oxadiazolo[3,4-d]pyrimidine-5,7-dione 1-oxides (2) are conveniently prepared in high yields by the oxidative intramolecular cyclization of 6-amino-5-nitro-1H-pyrimidine-2,4-diones (1) employing iodosylbenzene diacetate as an oxidant in the presence of lithium hydride. The generation of nitric oxide (NO) and NO-related species from 2
Micah J Bodner et al.
Organic letters, 11(16), 3606-3609 (2009-07-21)
Efficient syntheses of N-acetyl thienamycin and epithienamycin A in their readily deprotected form are reported where three contiguous stereocenters are established in a single catalytic asymmetric azetidinone-forming reaction. These examples are a template for synthesizing C-5/C-6 cis or trans carbapenems
N Singh et al.
Biochemical and biophysical research communications, 131(2), 786-792 (1985-09-16)
The acetyl transacylase activity of the fatty acid synthase from yeast has been investigated using p-nitrophenylthiol acetate. The chromophoric nature of the nitrophenylthiol moiety affords a convenient spectrophotometric assay for the transacylase function as well as a means to investigate
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.