추천 제품
Grade
reagent grade
Quality Level
vapor density
4.4 (vs air)
vapor pressure
150 mmHg ( 20 °C)
분석
98%
반응 적합성
reagent type: oxidant
불순물
≤1.0% phosgene content
refractive index
n20/D 1.429 (lit.)
bp
62-65 °C (lit.)
mp
−10-−8 °C (lit.)
density
1.5 g/mL at 20 °C (lit.)
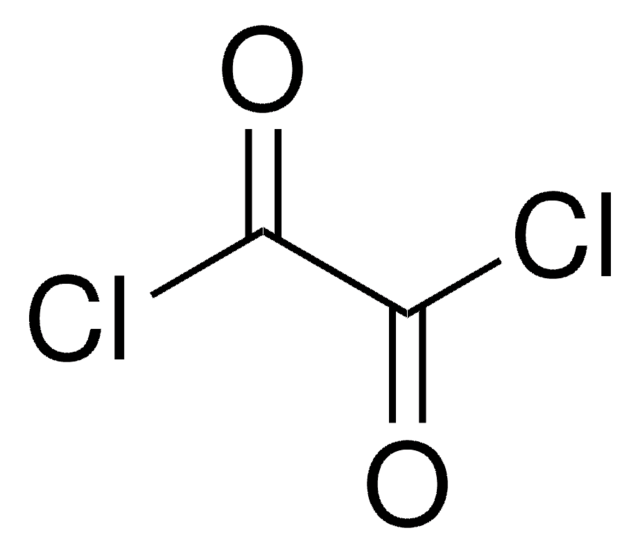
SMILES string
ClC(=O)C(Cl)=O
InChI
1S/C2Cl2O2/c3-1(5)2(4)6
InChI key
CTSLXHKWHWQRSH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Oxalyl chloride is a versatile reagent in various chemical transformations, such as chlorination, dichlorination, oxidation, reduction, dehydration, decarboxylation, formylation, and ring cleavage of epoxides.
애플리케이션
Oxalyl chloride can be used as an oxidizing agent:
- To synthesize β, β′-diketodithioethers from β, β′-dihydroxydithioethers via Swern oxidation.
- In the DMSO-catalyzed Swern oxidation of primary amides or aldoximes to nitriles in the presence of triethylamine as a base.
- In the Moffatt-Swern oxidation of aryl allylic alcohols to halogenated unsaturated ketones in the presence of triethylamine.
Reactant involved in:
- Synthesis of N-heterocyclic ynones and ynediones, used to activate carboxylic acids
- Chlorination and halogenation
- Three-component [3+2] cycloadditions
- Reactions with organostannanes
- Synthesis of cyclopentenones
- Carbonylations, used as a carbonyl synthon
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B - Water-react 1
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Faceshields, Gloves, Goggles
이미 열람한 고객
Oxalyl chloride as a practical carbon monoxide source for carbonylation reactions.
Hansen SVF and Ulven T.
Organic Letters, 17(11), 2832-2835 (2015)
A direct access to α-diones from oxalyl chloride.
Babudri F, et al.
Tetrahedron Letters, 36(40), 7305-7308 (1995)
Oxalyl halides: Part II. Vibrational spectra and assignments for oxalyl fluoride and oxalyl chloride fluoride.
Hencher JL and King GW.
Journal of Molecular Spectroscopy, 16(1), 168-178 (1965)
Systematic survey of positive chlorine sources in the asymmetric Appel reaction: oxalyl chloride as a new phosphine activator.
Rajendran KV, et al.
Tetrahedron Letters, 54(51), 7009-7012 (2013)
Tsutomu Kimura et al.
Chemical communications (Cambridge, England), (32)(32), 4077-4079 (2005-08-11)
Reaction of diastereomerically pure phosphinoselenoic acid salts with oxalyl chloride leads to enantiomerically pure P-chiral phosphinoselenoic chlorides with inversion of configuration at phosphorus; one of these chlorides is converted to a phosphinoselenothioic acid salt with a high degree of enantioselectivity.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.