310670
Oxalyl chloride solution
2.0 M in methylene chloride
동의어(들):
Dichlorooxalic acid, Ethanedioyl chloride, Oxalic acid chloride, Oxalic acid dichloride, Oxalic dichloride, Oxaloyl chloride, Oxaloyl dichloride, Oxalyl dichloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
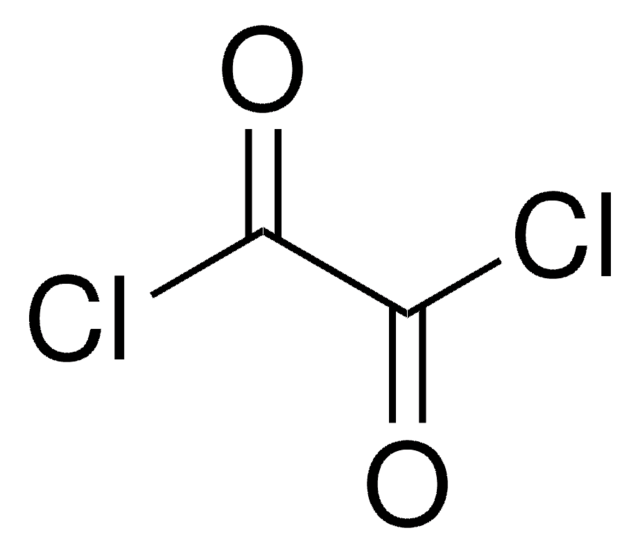
Linear Formula:
ClCOCOCl
CAS Number:
Molecular Weight:
126.93
Beilstein:
1361988
MDL number:
UNSPSC 코드:
12352106
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Oxalyl chloride is generally used as a dimethyl sulfoxide activator and a chlorinating agent to convert carboxylic acid to acid chlorides.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Synthesis and characterization of highly soluble and heat stable new poly (amide-ether) s containing pyridine rings in the main chain.
Banihashemi A and Vakili MR
e-Polymers, 8(1) (2008)
Catalytic syntheses of N-heterocyclic ynones and ynediones by in situ activation of carboxylic acids with oxalyl chloride.
Christina Boersch et al.
Angewandte Chemie (International ed. in English), 50(44), 10448-10452 (2011-09-13)
Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide" activated" by oxalyl chloride.
Mancuso AJ, et al.
The Journal of Organic Chemistry, 43(12), 2480-2482 (1978)
Benoît Heurtaux et al.
The Journal of organic chemistry, 70(4), 1474-1477 (2005-02-12)
[reaction: see text] Several natural pulvinic acids were synthesized. Silyl ketene acetals derived from methyl arylacetates (4 equiv) reacted with oxalyl chloride at -78 degrees C, without the need of adding a catalyst. After treatment of the crude diketones with
Peter J Manley et al.
Organic letters, 4(18), 3127-3129 (2002-08-31)
[reaction: see text] A mild, practical, one-pot method for the generation of imidoyl chlorides and their subsequent in situ reaction with pyridine-1-oxides is described. The imidoyl chlorides were formed from the reaction of secondary amides with a stoichiometric amount of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.