추천 제품
vapor density
4.4 (vs air)
Quality Level
vapor pressure
150 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
≥99%
형태
liquid
반응 적합성
reagent type: oxidant
불순물
<10 ppb Heavy metals
색상
APHA: 0-150
refractive index
n20/D 1.429 (lit.)
bp
62-65 °C (lit.)
mp
−10-−8 °C (lit.)
density
1.5 g/mL at 20 °C (lit.)
작용기
acyl chloride
SMILES string
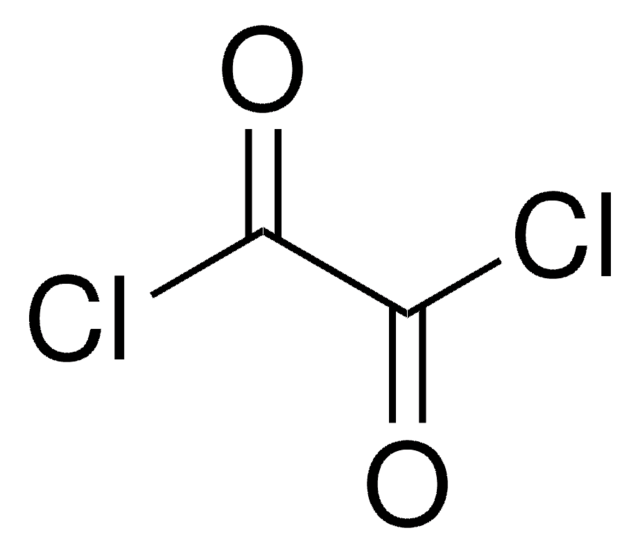
ClC(=O)C(Cl)=O
InChI
1S/C2Cl2O2/c3-1(5)2(4)6
InChI key
CTSLXHKWHWQRSH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Oxalyl chloride is a commonly used chlorinating reagent that can be prepared by the reaction of oxalic acid and phosphorus pentachloride.
애플리케이션
Oxalyl chloride may be used in the following processes:
- Preparation of Mosher′s acid chloride by reacting with Mosher′s acid in the presence of DMF.
- Activation of dimethyl sulfoxide for use in the oxidation of long-chain alcohols to carbonyls.
- Activation of α-keto carboxylic acids and N-heterocyclic carboxylic acids for alkynylation to form ynediones and N-heterocyclic ynones, respectively.
Suitable for the synthesis of acid chlorides used to produce liquid crystals.
Reactant involved in:
- Synthesis of N-heterocyclic ynones and ynediones, used to activate carboxylic acids
- Chlorination and halogenation
- Three-component [3+2] cycloadditions
- Reactions with organostannanes
- Synthesis of cyclopentenones
- Carbonylations, used as a carbonyl synthon
포장
The 5g, 25g and 100g units sold in the US are packaged in ampules.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - Water-react 1
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point (°F)
51.8 °F - closed cup
Flash Point (°C)
11.0 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles
이미 열람한 고객
Catalytic Syntheses of N-Heterocyclic Ynones and Ynediones by In Situ Activation of Carboxylic Acids with Oxalyl Chloride.
Boersch C, et al.
Angewandte Chemie (International Edition in English), 50(44), 10448-10452 (2011)
A simple method for the microscale preparation of Mosher's acid chloride.
Ward DE and Rhee CK.
Tetrahedron Letters, 32(49), 7165-7166 (1991)
Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide" activated" by oxalyl chloride.
Mancuso AJ, et al.
The Journal of Organic Chemistry, 43(12), 2480-2482 (1978)
Tsutomu Kimura et al.
Chemical communications (Cambridge, England), (32)(32), 4077-4079 (2005-08-11)
Reaction of diastereomerically pure phosphinoselenoic acid salts with oxalyl chloride leads to enantiomerically pure P-chiral phosphinoselenoic chlorides with inversion of configuration at phosphorus; one of these chlorides is converted to a phosphinoselenothioic acid salt with a high degree of enantioselectivity.
Peter J Manley et al.
Organic letters, 4(18), 3127-3129 (2002-08-31)
[reaction: see text] A mild, practical, one-pot method for the generation of imidoyl chlorides and their subsequent in situ reaction with pyridine-1-oxides is described. The imidoyl chlorides were formed from the reaction of secondary amides with a stoichiometric amount of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.