모든 사진(3)
About This Item
실험식(Hill 표기법):
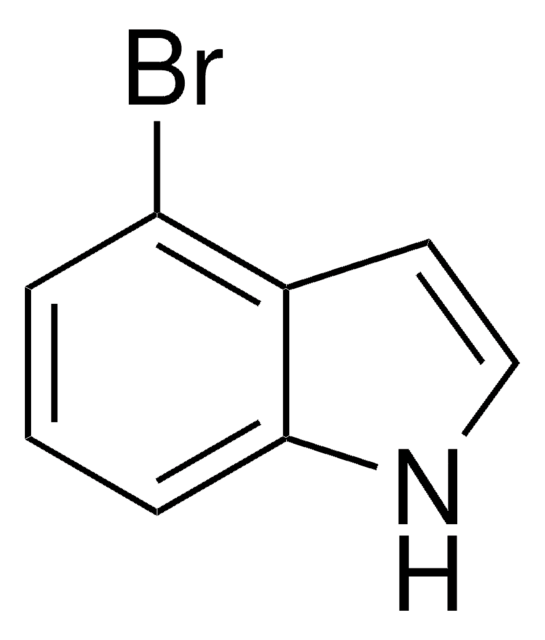
C8H6BrN
CAS Number:
Molecular Weight:
196.04
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
96%
양식
solid
mp
41-44 °C (lit.)
작용기
bromo
SMILES string
Brc1cccc2cc[nH]c12
InChI
1S/C8H6BrN/c9-7-3-1-2-6-4-5-10-8(6)7/h1-5,10H
InChI key
RDSVSEFWZUWZHW-UHFFFAOYSA-N
일반 설명
7-Bromoindole is a 7-substituted indole derivative. Its synthesis from 7-bromoindole-2-carboxylic acid has been reported. It has been reported to reduce the production of staphyloxanthin in Staphylococcus aureus.
애플리케이션
7-Bromoindole may be used in the synthesis of the following:
- indole
- dyestuffs
- 8-bromocarboline
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Zhiqian Wang et al.
Tetrahedron letters, 53(5), 477-479 (2012-05-01)
A novel MCAP-cycloaddition sequence has been applied to the facile synthesis of β-carboline intermediates to gain rapid access to novel derivatives of yohimbine-like and corynanthe-like compounds that may be easily diversified by cross-coupling reactions and N-derivatizations to generate small compound
Total synthesis of indoles from Tricholoma species via Bartoli/heteroaryl radical methodologies.
A Dobbs
The Journal of organic chemistry, 66(2), 638-641 (2001-06-30)
The structure of monobrominated ethyl indole-3-carboxylate and the preparation of 7-bromoindole.
Leggetter BE and Brown RK.
Canadian Journal of Chemistry, 38(9), 1467-1471 (1960)
Jin-Hyung Lee et al.
Applied microbiology and biotechnology, 97(10), 4543-4552 (2013-01-16)
Human pathogens can readily develop drug resistance due to the long-term use of antibiotics that mostly inhibit bacterial growth. Unlike antibiotics, antivirulence compounds diminish bacterial virulence without affecting cell viability and thus, may not lead to drug resistance. Staphylococcus aureus
J Y Kim et al.
Letters in applied microbiology, 41(2), 163-168 (2005-07-22)
To establish multicomponent phenol hydroxylases (mPHs) as novel biocatalysts for producing dyestuffs and hydroxyindoles such as 7-hydroxyindole (7-HI) from indole and its derivatives. We have isolated Pseudomonas sp. KL33, which possesses a phenol degradation pathway similar to that found in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.