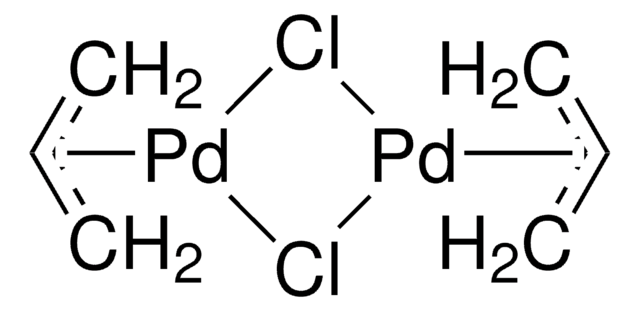
720526
Palladium(π-cinnamyl) chloride dimer
97%
동의어(들):
Bis[cinnamyl palladium(II) chloride], Palladium(1-phenylallyl)chloride dimer, [(Cinnamyl)PdCl]2, [Pd(1-phenylallyl)Cl]2
About This Item
추천 제품
Quality Level
분석
97%
양식
solid
반응 적합성
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
218-220 °C
저장 온도
2-8°C
SMILES string
Cl[Pd].Cl[Pd].[CH2][CH][CH]c1ccccc1.[CH2][CH][CH]c2ccccc2
InChI
1S/2C9H9.2ClH.2Pd/c2*1-2-6-9-7-4-3-5-8-9;;;;/h2*2-8H,1H2;2*1H;;/q;;;;2*+1/p-2
InChI key
SHWAPUDBEQXXLQ-UHFFFAOYSA-L
애플리케이션
- Ammonia cross-coupling reactions to synthesize arylamines.
- Conversion of aryl triflates to fluorides.
It can also be used as a source of palladium in the asymmetric α-arylation of amides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![5-(Di-tert-butylphosphino)-1′, 3′, 5′-triphenyl-1′H-[1,4′]bipyrazole 97%](/deepweb/assets/sigmaaldrich/product/structures/137/599/8b2f4b58-3384-40aa-9295-0887f7985525/640/8b2f4b58-3384-40aa-9295-0887f7985525.png)




![5-[Di(1-adamantyl)phosphino]-1′,3′,5′-triphenyl-1′H-[1,4′]bipyrazole 97%](/deepweb/assets/sigmaaldrich/product/structures/334/020/2da77724-fc86-4d88-bc93-0b43ae777daa/640/2da77724-fc86-4d88-bc93-0b43ae777daa.png)

![Bis[tris(2-methylphenyl)phosphine]palladium 97%](/deepweb/assets/sigmaaldrich/product/structures/340/188/b35c332e-1358-43d9-941c-3c7a410e30f7/640/b35c332e-1358-43d9-941c-3c7a410e30f7.png)