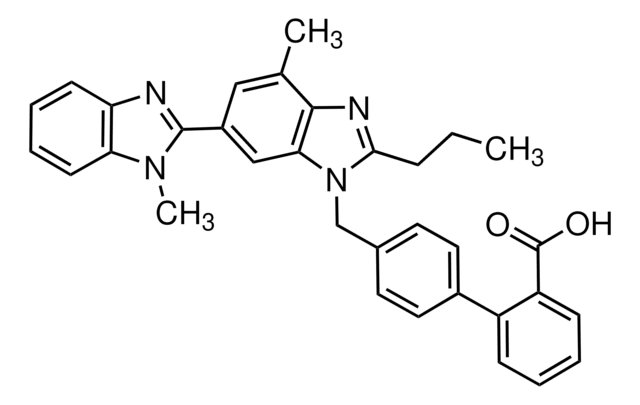
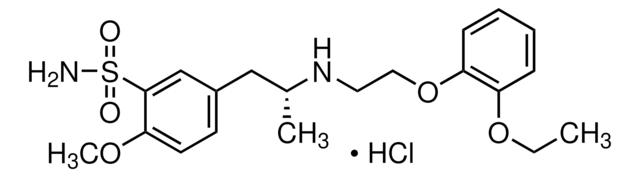
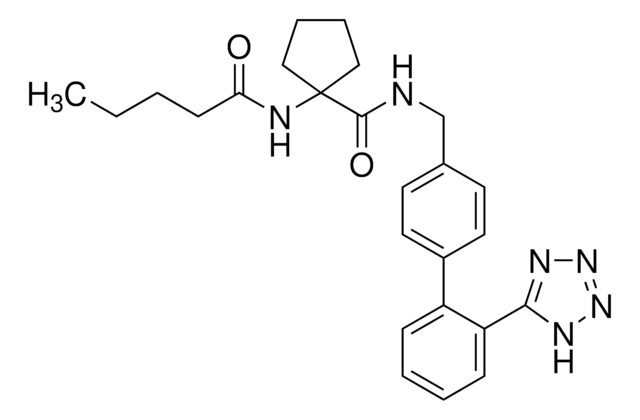
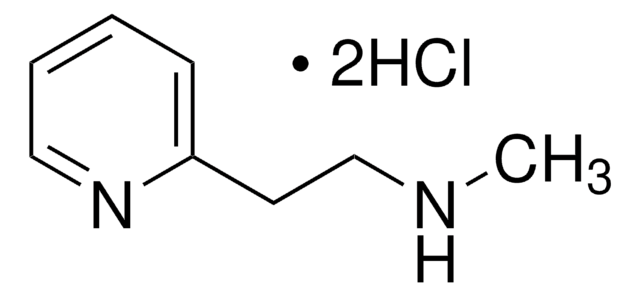
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. Y0001719
traceable to USP 1606015
API family
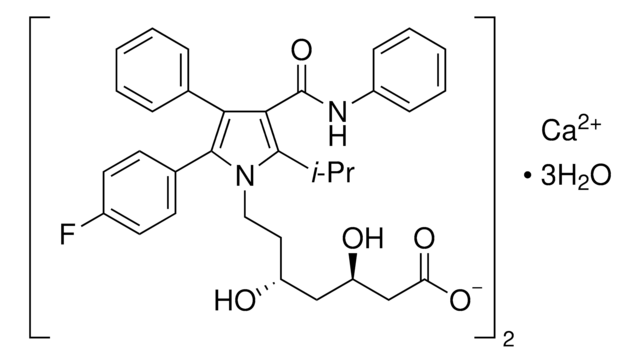
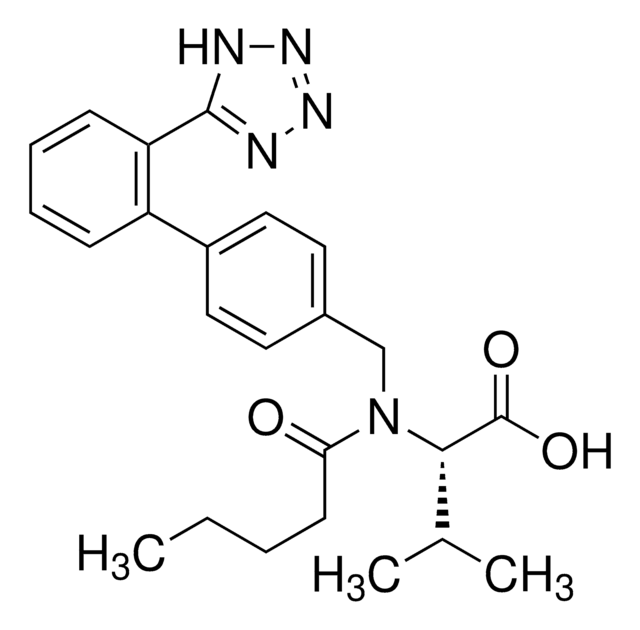
rosuvastatin
형태
powder
CofA
current certificate can be downloaded
포장
pkg of 1 g
응용 분야
pharmaceutical
저장 온도
-10 to -25°C
InChI
1S/2C22H28FN3O6S.Ca/c2*1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32;/h2*5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30);/q;;+2/p-2/b2*10-9+;/t2*16-,17-;/m11./s1
InChI key
LALFOYNTGMUKGG-BGRFNVSISA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Rosuvastatin Calcium, an HMG-CoA reductase inhibitor, belongs to the statins class of pharmaceuticals and is used for the treatment of hypercholesterolemia.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Rosuvastatin Calcium, an HMG-CoA reductase inhibitor, belongs to the statins class of pharmaceuticals and is used for the treatment of hypercholesterolemia.
애플리케이션
This pharmaceutical secondary standard can also be used as follows:
- Spectrophotometric estimation of rosuvastatin calcium in its pure form and tablet formulations
- Determination of rosuvastatin calcium by square-wave voltammetry using a boron-doped diamond electrode in two tablet samples and biological fluid samples of human urine and serum
- Simultaneous quantification of rosuvastatin and amlodipine by high-performance liquid chromatography (HPLC) in combined pharmaceutical formulations
- Determination of rosuvastatin calcium and related substances in tablets of rosuvastatin using a reversed-phase high-performance liquid chromatographic (RP-HPLC) method
분석 메모
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
각주
To see an example of a Certificate of Analysis for this material enter LRAC2081 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
추천 제품
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Spectrophotometric determination of rosuvastatin calcium in pure form and pharmaceutical formulations by the oxidation using iodine and formation triiodide complex in acetonitrile
Ramadan AA, et al.
International Journal of Pharmacy and Pharmaceutical Sciences, 6, 579-585 (2014)
Square-wave voltammetric determination of rosuvastatin calcium in pharmaceutical and biological fluid samples using a cathodically pretreated boron-doped diamond electrode
Silva TA, et al.
Diamond and Related Materials, 58, 103-109 (2015)
Simultaneous estimation of rosuvastatin and amlodipine in pharmaceutical formulations using stability indicating HPLC method
Ashfaq M, et al.
Brazilian Journal of Pharmaceutical Sciences , 50, 629-638 (2014)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.