추천 제품
Grade
pharmaceutical primary standard
API family
indinavir
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
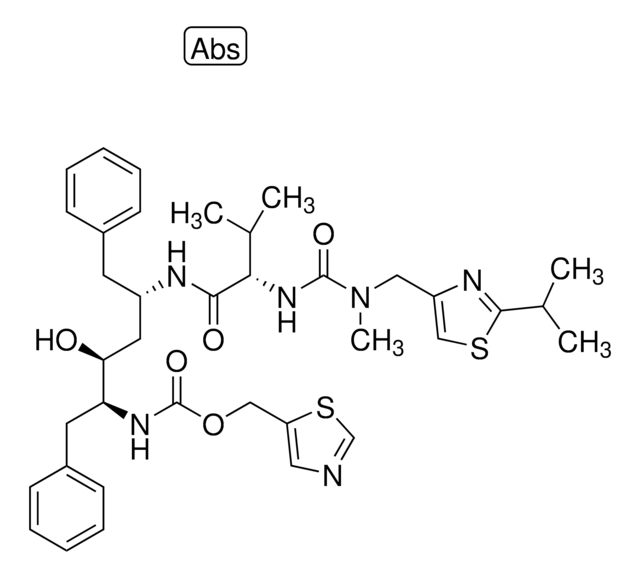
SMILES string
N4([C@@H](CN(CC4)Cc5cnccc5)C(=O)NC(C)(C)C)C[C@@H](O)C[C@@H](Cc3ccccc3)C(=O)N[C@@H]1[C@@H](Cc2c1cccc2)O.O
InChI
1S/C36H47N5O4.H2O/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43;/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45);1H2/t28-,29+,31+,32-,33+;/m1./s1
InChI key
XTYSXGHMTNTKFH-BDEHJDMKSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Indinavir EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Julieta C Imperiale et al.
Drug development and industrial pharmacy, 40(12), 1607-1615 (2013-09-21)
This work investigated the production of pure indinavir free base nanoparticles by a supercritical anti-solvent method to improve the drug dissolution in intestine-like medium. To increase the dissolution of the drug by means of a supercritical fluid processing method. Acetone
Dario Akaberi et al.
Journal of biomolecular structure & dynamics, 38(18), 5526-5536 (2019-12-28)
Zika virus (ZIKV) is an emerging mosquito-borne flavivirus and infection by ZIKV Asian lineage is known to cause fetal brain anomalies and Guillain-Barrés syndrome. The WHO declared ZIKV a global public health emergency in 2016. However, currently neither vaccines nor
Oh-Kyung Kwon et al.
Virology journal, 12, 53-53 (2015-04-17)
In South Korea, about 20 types of antiretroviral drugs are used in the treatment of patients with human immunodeficiency virus/acquired immune deficiency syndrome. Since 2010, raltegravir, etravirine, and darunavir have been spotlighted as new drugs for highly active antiretroviral therapy
Camille B Blake et al.
American journal of physiology. Regulatory, integrative and comparative physiology, 307(6), R711-R720 (2014-07-06)
Pathologies in which insulin is dysregulated, including diabetes, can disrupt central vagal circuitry, leading to gastrointestinal and other autonomic dysfunction. Insulin affects whole body metabolism through central mechanisms and is transported into the brain stem dorsal motor nucleus of the
Yasuo Uchida et al.
The Journal of pharmacology and experimental therapeutics, 350(3), 578-588 (2014-06-21)
The aim of this study was to investigate whether in vivo drug distribution in brain in monkeys can be reconstructed by integrating four factors: protein expression levels of P-glycoprotein (P-gp)/multidrug resistance protein 1 at the blood-brain barrier (BBB), in vitro
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.