Y0000896
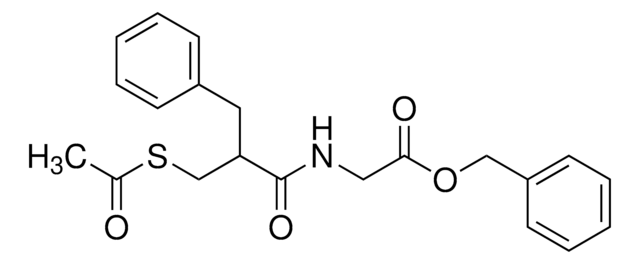
Racecadotril
European Pharmacopoeia (EP) Reference Standard
동의어(들):
(RS)-Benzyl N-[3-(acetylthio)-2-benzylpropanoyl]glycinate, (±)-Acetorphan, Acetorphan, Cadotril, Redotil, Tiorfan, Zedott
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C21H23NO4S
CAS Number:
Molecular Weight:
385.48
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
racecadotril
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
CC(=O)SCC(Cc1ccccc1)C(=O)NCC(=O)OCc2ccccc2
InChI
1S/C21H23NO4S/c1-16(23)27-15-19(12-17-8-4-2-5-9-17)21(25)22-13-20(24)26-14-18-10-6-3-7-11-18/h2-11,19H,12-15H2,1H3,(H,22,25)
InChI key
ODUOJXZPIYUATO-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Racecadotril EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
Neutral endopeptidase inhibitor; antidiarrheal
Racecadotril is a neutral endopeptidase inhibitor, an antidiarrheal drug which acts as a peripherally acting enkephalinase inhibitor.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
J M Lecomte
International journal of antimicrobial agents, 14(1), 81-87 (2000-03-16)
Since preclinical studies had indicated the potential efficacy and tolerability of racecadotril for the treatment of diarrhoea in man, a series of studies was carried out to assess the clinical effects of racecadotril. These studies were also designed to evaluate
Ramón Tormo et al.
Acta paediatrica (Oslo, Norway : 1992), 97(8), 1008-1015 (2008-05-09)
In developing countries acute infectious diarrhoea remains one of the leading causes of death among young children, especially those under 1 year of age. In contrast, in industrialized nations the death rate is very low, although the disease is an
J I Emparanza Knörr et al.
Anales de pediatria (Barcelona, Spain : 2003), 69(5), 432-438 (2009-01-09)
To estimate, through a systematic review of the literature, the efficacy of racecadotril in the treatment of acute diarrhoea. Randomised trials carried out in children comparing racecadotril with placebo in terms of diarrhoea recovery, stools output and adverse effects were
A J Matheson et al.
Drugs, 59(4), 829-835 (2000-05-10)
Racecadotril is an oral enkephalinase inhibitor used in the treatment of acute diarrhoea. It prevents the degradation of endogenous opioids (enkephalins), thereby reducing hypersecretion of water and electrolytes into the intestinal lumen. In a randomised double-blind study in 6 adult
H Szajewska et al.
Alimentary pharmacology & therapeutics, 26(6), 807-813 (2007-09-05)
Racecadotril (acetorphan) is an antisecretory drug that exerts its antidiarrhoeal effects by inhibiting intestinal enkephalinase. To summarize studies testing the efficacy and safety of racecadotril for treating children with acute gastroenteritis. Reports were gathered by searching electronic databases MEDLINE, EMBASE
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.